- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
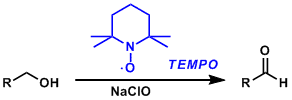
2,2,6,6-Tetramethylpiperidine-1-oxyl radical (TEMPO) is a stable and commercially available organic free radical reagent used to oxidize primary alcohols to aldehydes. It is usually used in substoichiometric amounts in combination with a stoichiometric reoxidant such as sodium hypochlorite and PhI(OAc)2. Selective oxidation of primary alcohols is possible since secondary alcohols are much less reactive under these conditions.
-
General References
・Lebelev, O. L.; Kazarnovskii, S. N. Zhur. Obshch. Khim. 1960, 30, 1631.
・de Nooy, A. E. J.; Besemer, A. C.; van Bekkum, H. Synthesis 1996, 1153. DOI: 10.1055/s-1996-4369
-
Reaction Mechanism
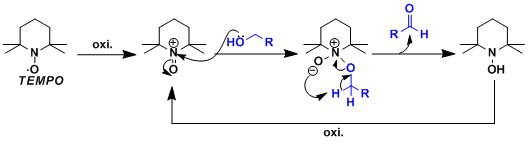
The N-oxoammonium cation produced by the oxidation of TEMPO works as the active oxidizing species.
-
Examples
The TEMPO oxidation in the synthesis of kuehneromycin A.[1]

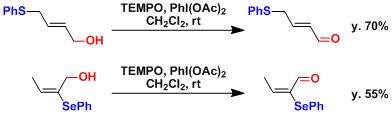
Allylic alcohols are especially reactive that they can be oxidized selectively in the presence of other oxidizable groups containing sulfur and selenium.[2]
An example where selective oxidation of the primary hydroxyl group is achieved under biphasic conditions.[3]
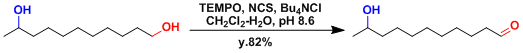
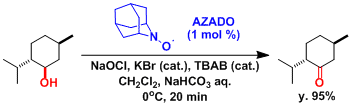
The AZADO reagent is less sterically hindered and more reactive than TEMPO. Congested alcohols can be oxidized using this reagent.[4]
-
Experimental Procedure
The TEMPO oxidation using PhI(OAc)2 as a reoxidant.[5]

A 250-mL round-bottomed flask equipped with a Teflon-coated magnetic stirring bar is charged with the following order of the reagents: acetonitrile (28 mL), (Z)-3,7-dimethyl-2,6-octadien-1-ol (nerol) (5.70 mL, 32.5 mmol), aqueous pH 7.0 buffer solution (8 mL), 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) (490 mg, 3.24 mmol), and iodobenzene diacetate (IBD) (11.49 g, 35.71 mmol). The reaction mixture is stirred at 0 °C until nerol is no longer detectable by TLC analysis. The reaction mixture is diluted with diethyl ether (100 mL) and transferred to a 500-mL separatory funnel. The orange mixture is washed with saturated aqueous sodium thiosulfate (2 x 50 mL). The aqueous phase is separated and extracted with diethyl ether (3 x 35 mL). The combined organic layers are washed with saturated aqueous sodium hydrogen carbonate (40 mL) and then with saturated aqueous sodium chloride (40 mL). The organic layer is dried over anhydrous sodium sulfate, filtered, and concentrated with a rotary evaporator (35 °C, 70 mmHg). The residue is purified by column chromatography on silica gel, using a 1:9 mixture of diethyl ether and hexanes as eluent to afford 4.30–4.39 g (87–89%) of (Z)-3,7-dimethyl-2,6-octadien-1-al (neral) as a colorless oil.
-
Experimental Tips
-
References
[1] Jauch, J. Angew. Chem., Int. Ed. 2000, 39, 2764. [abstract]
[2] De Mico, A.; Margarita, R.; Parlanti, L.; Vescovi, A.; Piancatelli, G. J. Org. Chem. 1997, 62, 6974. DOI: 10.1021/jo971046m
[3] Einhorn, J.; Einhorn, C.; Ratajczak, F.; Pierre, J.-L. J. Org. Chem. 1996, 61, 7452. DOI: 10.1021/jo9609790
[4] Shibuya, M.; Tomizawa, M.; Suzuki, I.; Iwabuchi, Y. J. Am. Chem. Soc. 2006, 128, 8412. DOI: 10.1021/ja0620336
[5] Piancatelli,G.; Leonelli, F. Org. Synth. 2006, 83, 18. [PDF]
-
Related Books
[amazonjs asin=”0198556640″ locale=”US” title=”Oxidation and Reduction in Organic Synthesis (Oxford Chemistry Primers, 6)”]
[amazonjs asin=”0387236074″ locale=”US” title=”Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice (Basic Reactions in Organic Synthesis)”]