- Popularity
- User-friendly
- Reliability
- Criteria #4
- Criteria #5
-
Characteristics
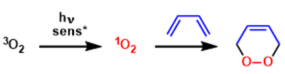
Since molecular oxygen exists in a triplet ground state (3O2),the spin conservation rule restricts its transforma- tions to radical-type reactions, which are normally quite unselective. In contrast, in its singlet excited state, 02 circumvents the problem of spin-forbidden reactions and, consequently, selective novel cycloadditions become possible, which complement in a useful way the activation of dioxygen by metal complexes.
The existence of singlet molecular oxygen has been recognized since 1924.
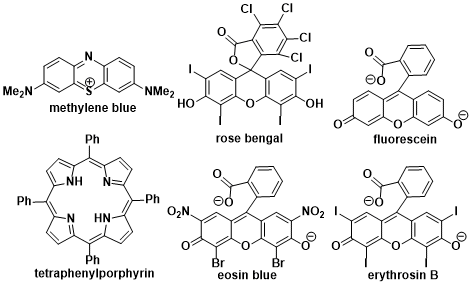
Besides the chemical generation of singlet oxygen photosensitization of triplet molecular oxygen by visible light under the optimal photosensitizer (methylene blue, rose bengal and fluorescein etc.. are shown below) is by far the most common and convenient method for in situ generation of this highly reactive, yet selective oxidant.
One can roughly categorize the reactions of singlet oxygen into two classes: (1) cycloaddition to form cyclic peroxides and (2) ene reactions to form hydroperoxide.
-
Literature reference
[a] Wasserman,H. H.; Murray, R. W. “SingletOxygen, A Series of Monographs”;Academic Press: New York, 1979;Vol. 40, p xiii.
<review>
・Balci, M. Chem. Rev. 1981, 81, 91. DOI: 10.1021/cr00041a005
・Prein, M.; Adam, W. Angew. Chem. Int. Ed. Engl. 1996, 35, 477. DOI: 10.1002/anie.199604771
・Stratakis, M.; Orfanopoulos, M. Tetrahedron 2000, 56, 1595. DOI:10.1016/S0040-4020(99)00950-3
・Clennan, E. L. Tetrahedron 2000, 56, 9151. DOI: 10.1016/S0040-4020(00)00794-8
・DeRosa, M. C.; Crutchley, R. J. Coord Chem. Rev. 2002, 233-234, 351. DOI:10.1016/S0010-8545(02)00034-6
・Clennan, E. L.; Pace, A. Tetrahedron 2005, 61, 6665. DOI:10.1016/j.tet.2005.04.017
・Margaros, I.; Montagnon, T.; Tofi, M.; Pavalakos, E.; Vassilikogiannakis, G. Tetrahedron 2006, 62, 5308. DOI:10.1016/j.tet.2006.01.110
・Montagnon, T.; Tofi, M.; Vassilikogiannakis, G. Acc. Chem. Res. 2008, 41, 1001. DOI:10.1021/ar800023v
・Hoffmann, N. Chem. Rev. 2008, 108, 1052. DOI: 10.1021/cr0680336
-
Reaction mechanism
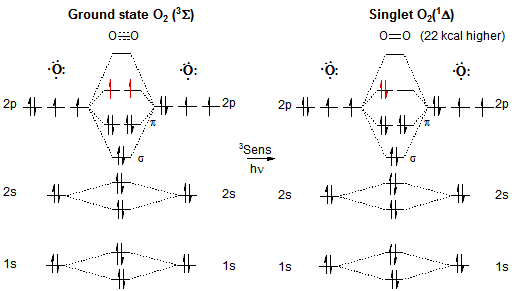
The singlet spin multiplicity significantly increases reactivity with respect to triplet (ground state) oxygen.
In photo-oxygenation reactions, singlet oxygen is generated by sensitization. Due to the low energy difference between these two species 3sigma and 1delta (22 kcal‚mol-1), dyes can be used as a sensitizer possessing a low excitation energy.
(excerpt from chem.wisc.edu)
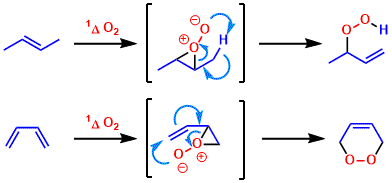
The first intermediate resulting from an interaction of singlet oxygen with an epoxide, alkene, and diene are an exciplex (excited complex). Various other intermediates are discussed in the literature. The most significant ones used to explain final product formation are shown below (only showcases alkenes and dienes in here).
Main reaction pathways result from this intermediate leading to dioxetanes (from epoxide), hydroperoxides (from alkene), and endoperoxides (from diene).
-
Example of reactions
In the total synthesis of Norzoanthamine by Miyashita et al, photosensitized oxidation of furan by singlet oxygen was employed.[1a] The photosensitized oxidation of the furan was successfully performed according to the Katsumura protocol[1b] with a halogen lamp and rose bengal to afford the desired Z-gammna–keto- alpha ,– beta unsaturated silyl ester quantitatively, which was immediately converted to the stable methyl ester using tetrabutylammonium fluoride (TBAF) and iodomethane (MeI) in THF in 97% yield (two steps).
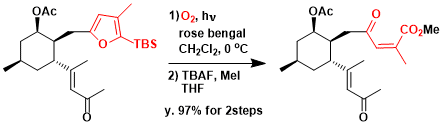
-
Bibliography
[1] (a) Miyashita, M.; Sasaki, M.; Hattori, I.; Sakai, M.; Tanino, K. Science 2004, 305, 495. DOI:10.1126/science.1098851 (b) Katsumura, S.; Hori, K.; Fujiwara, S.; Isoe, S. Tetrahedron Lett. 1985,26, 4625. doi:10.1016/S0040-4039(00)98769-0
-
Related Books
[amazonjs asin=”0121822206″ locale=”US” title=”Singlet Oxygen, UV-A and Ozone, Volume 319 (Methods in Enzymology)”][amazonjs asin=”B00DWHOJZU” locale=”US” title=”Photochemically-Generated Intermediates in Synthesis”][amazonjs asin=”1891389254″ locale=”US” title=”Modern Molecular Photochemistry of Organic Molecules”]
-
Related Links
・Singlet Oxygen - Wikipedia ・Singlet Oxygen in Organic Synthesis (Baran's group, PDF) ・Singlet Oxygen