- Popularity
- Reliability
- Versatility
- Criteria #4
- Criteria #5
-
Characteristics
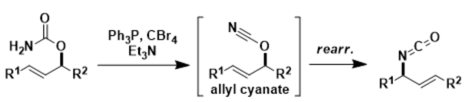
Ichikawa Allylcyanate rearrangement is [3.3] sigmatropic rearrangement of allyl cyanates to isocyanate. The rearrangement begins with dehydration of allyl carbamate by Appel conditions (CBr4, PPh3, Et3N), the resulting allyl cyanete immediately undergoes [3,3] bond reorganization to give rise to allyl isocyanate. Treatment of the resultant ally isocyanate with alcohol affords the corresponding carbamate.
This reaction is similar to Overman rearrangement, which allyl imidate undergoes [3.3]-sigmatropic rearrangement to form allyl trichloroacetamide xylene (140 °C). However, this reaction occurs smoothly at low temperature (less than 0 ºC) to provide the ally isocyanate.
-
Literature reference
・Ichikawa, Y. Synlett 1992, 238. DOI: 10.1055/s-1991-20691
<review>
・Ichikawa, Y. J. Org. Synth. Chem. Jpn. 2006, 64, 96.
・Ichikawa, Y. Synlett 2007, 2927. DOI: 10.1055/s-2007-992370
-
Reaction mechanism
Isomerization of the allyl cyanate is a concerted process involving a six-membered cyclic transition state with a high degree of [1,3]-chirality transfer.
-
Example of reactions
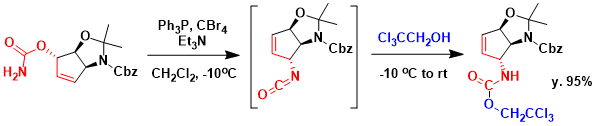
Ichikawa and coworkers demonstrated the nitrogen-substituted stereogenic center by [3.3] sigmatropic rearrangement of an allyl cyanate in the synthesis of Agelastatin A.[1] Dehydration of the carbamate with triphenylphosphine, carbon tetrabromide, and triethylamine in dichloromethane at – 10 ° C generated the allyl cyanate, which instantaneously rearranged into the allyl isocyanate via a concerted cyclic transition state. Then trapping of the isocyanate with 2,2,2-trichloroethanol gave the trichloroethoxy (Troc) carbamate in 95% yield.
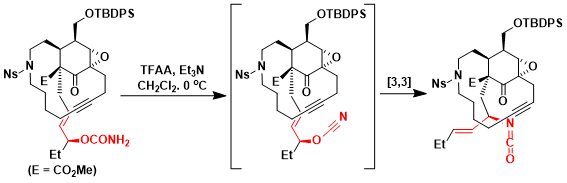
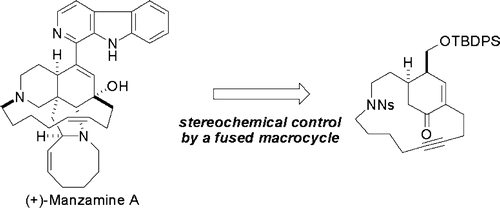
Fukuyama and coworkers utilized Ichikawa allylcyanate rearrangement for the total synthesis of (+)-manzamine A.[2] Upon dehydration of carbamate with TFAA and Et3N, the critical [3,3]-sigmatropic rearrangement proceeded even at 0 °C to give the corresponding isocyanate with complete control of the stereochemistry.
-
Bibliography
[1] Ichikawa, Y.; Yamaoka, T.; Nakano, K.; Kotsuki, H. Org. Lett. 2007, 9, 2989. DOI:10.1021/ol0709735
[2] “Total Synthesis of (+)-Manzamine A”
Toma,T.; Kita, Y.; Fukuyama, T. J. Am. Chem. Soc. 2010, 132, 10233. DOI:10.1021/ja103721s
A novel synthetic route to (+)-manzamine A was developed. It highlights an amazingly efficient construction of a highly strained 15-membered ring across a cyclohexenone ring with the aim of installing the requisite functionalities in a completely stereocontrolled manner. Other key features include a stereoselective Diels−Alder reaction of an optically active butenolide, construction of the 15-membered ring by intramolecular Mitsunobu reaction of a nosyl amide, [3,3]-sigmatropic rearrangement of allyl cyanate for stereoselective introduction of nitrogen functionality at a sterically congested position, and a ring-closing metathesis in the presence of labile functional groups.
-
Related Books
[amazonjs asin=”1230533753″ locale=”US” title=”Rearrangement reactions: Nazarov cyclization reaction, Sigmatropic reaction, Tiffeneau-Demjanov rearrangement, Alpha-ketol rearrangement, … rearrangement, Arndt-Eistert reaction”]