- Popularity
- Criteria #2
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
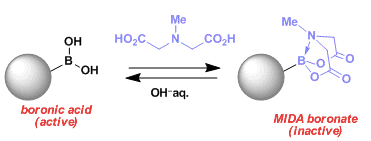
N-methyliminodiacetic acid (MIDA) is very useful protecting group for boronic acid and forms stable (more stable that the free acid) boronates which are readily prepared from the boronic acid.
Even though the lone electron pairs on the oxygen atoms of boronic acids and ester can provide partial stabilization of boron empty π orbital , a full protection that involves the coordinative sheilding of the empty π orbital from a heteroatom can dramatically deactivate the Lewis acidity of boronic acids. On the basis of this approach, Bruke and coworkers have discovered that MIDA could be used as an effective protecting group for boronic acids, allowing various reactions such as oxidation, cross-coupling reactions, and aldol reaction to be achieved in the presence of MIDA boronate.[a] In addition to being chemically inert in various conditions, MIDA boronate are also stable for chlomatography and are shelf-stable solids. Despite a protective ability associated with MIDA boronates, Burke and coworkers have successfully developed an approach that slowly releases boronic acids in situ into the reaction mixture for cross-coulong reactions (see earlier discussion). The slow release of these boronic acids suppresses side reactions such as protodeboronation and homocoupling reactions. This feature is especially beneficial during cross-coupling reactions of heteroaryl and alkenyl MIDA boronates.
-
Literature reference
[a] Gillis, E. P.; Burke, M. D. J. Am. Chem. Soc. 2007, 129, 6716. DOI: 10.1021/ja0716204
<review>
・ Gillis, E. P.; Burke, M. D. Aldirchimica Acta 2009, 42, 17. [PDF]
-
Example of reactions
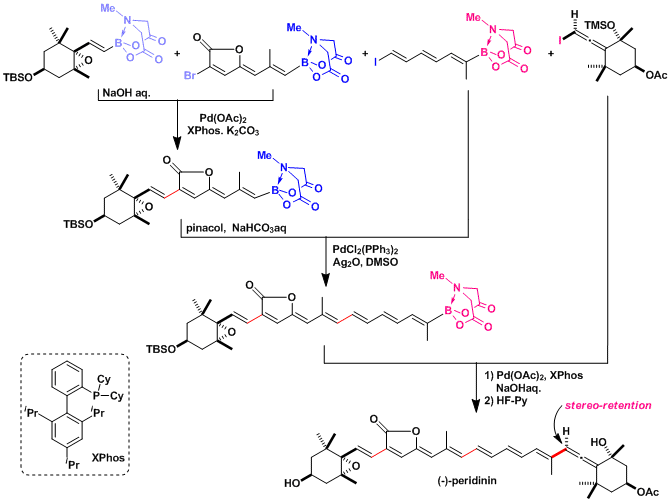
Total synthesis of (−)-Peridinin.
-
Bibliography
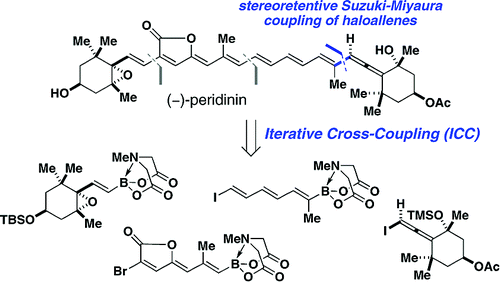
[1] “Stereoretentive Suzuki−Miyaura Coupling of Haloallenes Enables Fully Stereocontrolled Access to (−)-Peridinin”
Woerly, E. M.; Cherney, A. H.; Davis, E. K.; Burke, M. D. J. Am. Chem. Soc. 2010, 132, 6941. doi:10.1021/ja102721p
Stimulated by the substantial challenge of synthesizing the complex and sensitive stereogenic allene-containing core of (−)-peridinin, the first stereocontrolled coupling of haloallenes with boronic acids has been achieved. This new method and the principles that emerged during its development stand to enable the more efficient and flexible preparation of a wide range of natural products, pharmaceuticals, and intermediates that possess a stereogenic allene motif. This new reaction was harnessed to achieve the first completely stereocontrolled total synthesis of (−)-peridinin. This synthesis was accomplished using only one reaction iteratively to assemble four fully functionalized building blocks with complete stereoretention at each initial halide or boron-bearing carbon. This synthesis elevates the capacity of the iterative cross-coupling strategy to an unprecedented benchmark. Moreover, the efficient and highly modular nature of this synthesis promises to enable systematic dissection of the heretofore enigmatic structure/function relationships that underlie the protein-like antilipoperoxidant activities of this remarkable small molecule natural product.
-
Related Books
[amazonjs asin=”3527325980″ locale=”US” title=”Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials”][amazonjs asin=”0471697540″ locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”]
-
Related Links
・Organoborane (Wikipedia) ・Suzuki Reaction (Wikipedia)
・Burke Laboratory