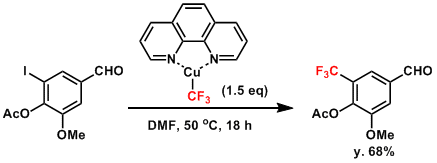- Simplicity
- Popularity
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
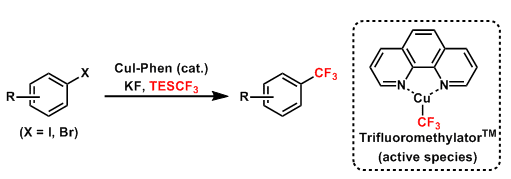
Amii trifluoromethylation is a the synthesis of trifluoromethylarenes from aryl/heteroaryliodides with CF3SiEt3 by Cu-catalsis.
Selective introduction of trifluoromethyl groups into aromatic compounds is of great importance in the medicinal, agricultural, and material sciences. Stoichiometric (sometimes, excess) amounts of copper reagents are required to complete these cross-coupling reactions. Amii and coworkers discovered that Cu(I)-diamine complexes were catalyzed the trifluoromethylation of aryl iodides. In the presence of a small amount of CuX (X = Cl, Br, I) and 1,10-phenanthroline (phen), the cross-coupling reactions of iodoarenes with trifluoromethylsilanes proceeded smoothly to afford trifluoromethylated aromatics in good yields.[a]
Afterwords, Hartwig developed the complex of phen and CuCF3, then it was commercialy available as trifluoromethylator [(Phen)Cu–CF3] from Sigma Aldrich.[b] It is an easily handled, thermally stable, single-component reagent for the trifluoromethylation of aryl iodides.
-
Literature reference
・ Kobayashi, Y.; Kumadaki, I. Tetrahedron Lett. 1969, 10, 4095. DOI:10.1016/S0040-4039(01)88624-X
・ Urata, H.; Fuchikami, T. Tetrahedron Lett. 1991, 32, 91. DOI:10.1016/S0040-4039(00)71226-3
・ Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600. DOI:10.1021/ja802946s
・ Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233. DOI:10.1021/om800794m
[a] Oishi, M.; Kondo, H.; Amii, H. Chem. Commun. 2009, 1909. DOI: 10.1039/b823249k
[b] Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem. Int. Ed. 2011, 50, 3793. DOI: 10.1002/anie.201100633
・ Tomashenko, O. A.; Escudero-Adan, E. C.; Belmonte, M. M.; Grushin, V. V. Angew. Chem. Int. Ed.2011, 50, 7655. DOI:10.1002/anie.201101577
・ Zanardi, .; Novikov, M. A.; Martin, E.; Benet-Buchholz, J.; Grusin, V. V. J. Am. Chem. Soc. 2011,133, 20901. DOI: 10.1021/ja2081026
・ Schareina, T.; Wu, X.-F.; Zapf, A.; Cotte, A.; Gotta, M.; Beeller, M. Top. Catal. 2012, 55, 426. DOI10.1007/s11244-012-9824-0
・ Fier, P. S.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 5524. DOI: 10.1021/ja301013h
<Palladium Catalysis>
・Kitazume, T.; Ishikawa, N. Chem. Lett. 1982, 137. DOI:10.1246/cl.1982.137
・Cho, E. J.; Senecal, T. D.; Kinzel, T.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. Science 2010, 328, 1679. DOI: 10.1126/science.1190524
<Review>
・Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475. DOI:10.1021/cr1004293
・Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470. DOI:10.1038/nature10108
・Liu, T.; Shen, Q. Eur. J. Org. Chem. 2012, 34, 6679. DOI: 10.1002/ejoc.201200648
・Garcia-Monforte, M. A.; Martinez-Salvador, S.; Menjon, B. Eur. J. Inorg. Chem. 2012, 4945. DOI:10.1002/ejic.201200620
・ Wu. X.-F.; Neumann, H.; Beller, M. Chem. Eur. J. 2012, 7, 1744. DOI: 10.1002/asia.201200211
・Jin, Z.; Hammond, G. B.; Xu, B. Aldrichimica Acta 2012, 45, 67. [PDF]
-
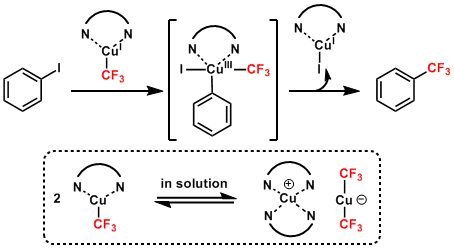
Reaction mechanism
The reaction mechanism still unclear, but a plausible mechanism is proposed as shown below. First, a fluoride ion reacts with CF3SiEt3 to give a trifluoromethyl anion (CF3–) species, which readily undergoes generation of trifluoromethylcopper “Cu–CF3” Next, sigma-bond metathesis between “Cu–CF3” and Ar–I (or oxidative addition of Ar–I bond) yields trifluoromethyl arenas and CuI. Diamine ligands in trifluoromethylated complexes would increase electron density at the metal centers by coordination and improve the nucleophilicity of the CF3 moieties in “Cu–CF3” in the second step.
-
Procedure
Example of the trifluoromethylation using trifluoromethylator.[1]
To a 20 mL vial equipped with a stir bar was added ArI (if solid, 0.50 mmol), trifluoromethylator (235 mg, 0.75 mmol, 1.5 equiv), and DMF (2.0 mL). Then ArI (if liquid, 0.50 mmol) was added, and the mixture was stirred at the indicated temperature in Scheme 4 (room temperature or 50 ºC). After 18 h, the stirring was stopped, and the reaction mixture was diluted with Et2O and filtered through a pad of Celite. The Celite pad was washed with Et2O. The combined filtrate was washed with 1m aqueous HCl, saturated aqueous NaHCO3 solution and brine, and dried over Na2SO4. After filtration and evaporation of the solvent, the crude mixture was purified by flash silica gel column chromatography using pentane/Et2O or pentane as eluent to give ArCF3.
-
Bibliography
[1] Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem. Int. Ed. 2011, 50, 3793. DOI: 10.1002/anie.201100633
-
Related Books
[amazonjs asin=”3838136209″ locale=”US” title=”Direct N-Trifluoromethylation: Direct Trifluoromethylation of Organonitrogen Compounds with Hypervalent Iodine Reagents”][amazonjs asin=”111807856X” locale=”US” title=”Efficient Preparations of Fluorine Compounds”]
-
Related Links