- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
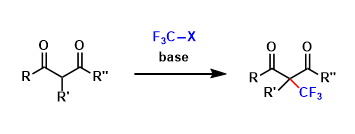
Fluorine and hydrogen are about the same size, but are electronically very different. The substitution of hydrogen atoms with fluorine atoms is a common way of tuning the electronic property of molecules without substantially affecting their size. In particular, trifluoromethyl (CF3) group is an important functional group in drug development.
The introduction of CF3 group is roughly divided into two approaches: nucleophilic conditions (the Ruppert-Prakash method) and electrophilic/free radical conditions.
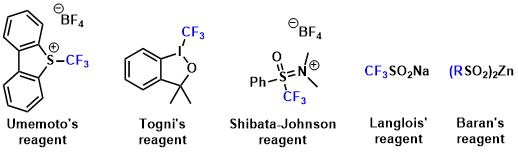
For the latter, many new useful reagents have been developed in the past few years. Representative examples are shown below.
-
General References
<General Reviews>
- Umemoto T. Chem. Rev. 1996,96, 1757. DOI: 10.1021/cr941149u
- Ma, J.-A.; Cahard, D. Chem. Rev. 2004, 104, 6119. DOI: 10.1021/cr030143e
- Ma, J.-A.; Cahard, D. J. Fluor. Chem. 2007, 128, 975. doi:10.1016/j.jfluchem.2007.04.026
- Shibata, N.; Matsnev, A.; Cahard, D. Beil. J. Org. Chem. 2010, 6, 65. doi:10.3762/bjoc.6.65
<Umemoto’s reagent>
- Umemoto T, Ishihara S. Tetrahedron Lett. 1990, 31, 3579. doi:10.1016/S0040-4039(00)94447-2
- Umemoto T, Ishihara S. J. Am. Chem. Soc. 1993, 115, 2156. DOI: 10.1021/ja00059a009
- Umemoto, T.; Adachi, K.; Ishihara, S. J. Org. Chem. 2007, 72, 6905. DOI: 10.1021/jo070896r
- Li, H. Synlett 2012, 2289. DOI: 10.1055/s-0032-1317176
<Togni’s reagent>
- Eisenberger, P.; Gischig, S.; Togni, A. Chem. Eur. J. 2006, 12, 2579. DOI: 10.1002/chem.200501052
- Kieltsch, I,; Eisenberger, P.; Togni, A. Angew. Chem. Int. Ed. 2007, 46, 754. DOI: 10.1002/anie.200603497
- Eisenberger, P.; Kieltsch, I.; Armanino, N.; Togni, A. Chem. Commun. 2008, 1575. DOI: 10.1039/B801424H
- Stanek, K.; Koller, R.; Togni, A. J. Org. Chem. 2008, 73, 7678. DOI: 10.1021/jo8014825
- Koller, R.; Stanek, K.; Stolz, D.; Aardoom, R.; Niedermann, K.; Togni, A. Angew. Chem. Int. Ed. 2009, 48, 4332. DOI: 10.1002/anie.200900974
- Eisenberger, P.; Kiltsch, I.; Koller, R.; Stanek, K.; Togni, A. Org. Synth. 2011, 88, 168. [PDF]
<Shibata-Johnson reagent>
- Noritake, S.; Shibata, N.; Nakamura, S.; Toru, T.; Shiro, M. Eur. J. Org. Chem. 2008, 3465. DOI: 10.1002/ejoc.200800419
<Langlois/Baran’s reagent>
- Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525. doi:10.1016/0040-4039(91)80524-A
- Ji, Y.; Bruecki, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. USA 2011, 108, 14411. doi: 10.1073/pnas.1109059108
- Fujiwara, Y.; Dixon, J.A.; Rodriguez, R.A.; Baxter, R.D.; Dixon, D.D.; Collins, M.R.; Blackmond, D.G.; Baran, P.S. J. Am. Chem. Soc. 2012, 134, 1494. DOI: 10.1021/ja211422g
- Fujiwara, Y.; Dixon, J.A.; O’Hara, F.; Daa Funder, E.; Dixon, D.D.; Rodriguez, R.A.; Baxter, R.D.; Herle, B.; Sach, N.; Collins, M.R.; Ishihara, Y.; Baran, P.S. Nature 2012, 492, 95. doi:10.1038/nature11680
- Ye, Y.; Kunzi, S. A.; Sanford, M. S. Org. Lett. 2012, 14, 4979. DOI: 10.1021/ol3022726
-
Reaction Mechanism
Since the carbon atom of CF3 group is surrounded by electronegative fluorine atoms, it is not particularly reactive towards substitution via backside attack (SN2 reaction).
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
Electrophilic Fluorination Reagent
-
Related Books
[amazonjs asin=”0895737051″ locale=”US” title=”Fluorine-Containing Molecules: Structure, Reactivity, Synthesis, and Applications (Molecular Structure and Energetics)”]
[amazonjs asin=”0471543705″ locale=”US” title=”Synthetic Fluorine Chemistry”]
[amazonjs asin=”352730617X” locale=”US” title=”Handbook of Fluorous Chemistry”]
-
External Links
Nucleophilic and Electrophilic Trifluoromethylation (PDF)
Trifluoromethylator(Sigma-Aldrich)
Trifluoromethylation – Wikipedia