- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
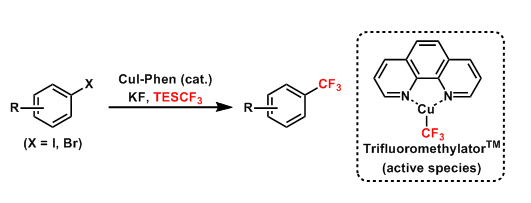
Aromatic halides can be trifluoromethylated by copper-catalyzed cross coupling developed by Amii. This procedure is applicable to the introduction of other perfluoroalkyl groups as well.
Close investigations into the active species by Hartwig in recent years have led to the development of (Phen)Cu-CF3, a thermally stable and easily handleable reagent now commercially available with the name TrifluoromethylatorTM.
-
General References
Kobayashi, Y.; Kumadaki, I. Tetrahedron Lett. 1969, 10, 4095. doi:10.1016/S0040-4039(01)88624-X
- Urata, H.; Fuchikami, T. Tetrahedron Lett. 1991, 32, 91. doi:10.1016/S0040-4039(00)71226-3
- Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600. doi:10.1021/ja802946s
- Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233. DOI: 10.1021/om800794m
- Oishi, M.; Kondo, H.; Amii, H. Chem. Commun. 2009, 1909. DOI: 10.1039/b823249k
- Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem. Int. Ed. 2011, 50, 3793. DOI: 10.1002/anie.201100633
- Tomashenko, O. A.; Escudero-Adan, E. C.; Belmonte, M. M.; Grushin, V. V. Angew. Chem. Int. Ed. 2011, 50, 7655. doi:10.1002/anie.201101577
- Zanardi, .; Novikov, M. A.; Martin, E.; Benet-Buchholz, J.; Grusin, V. V. J. Am. Chem. Soc. 2011, 133, 20901. DOI: 10.1021/ja2081026
- Schareina, T.; Wu, X.-F.; Zapf, A.; Cotte, A.; Gotta, M.; Beeller, M. Top. Catal. 2012, 55, 426. DOI 10.1007/s11244-012-9824-0
- Fier, P. S.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 5524. DOI: 10.1021/ja301013h
< Palladium Catalysis> - Kitazume, T.; Ishikawa, N. Chem. Lett. 1982, 137. doi:10.1246/cl.1982.137
- Cho, E. J.; Senecal, T. D.; Kinzel, T.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. Science 2010, 328, 1679. DOI: 10.1126/science.1190524
<Review>
- Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475. doi:10.1021/cr1004293
- Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470. doi:10.1038/nature10108
- Liu, T.; Shen, Q. Eur. J. Org. Chem. 2012, 34, 6679. DOI: 10.1002/ejoc.201200648
- Garcia-Monforte, M. A.; Martinez-Salvador, S.; Menjon, B. Eur. J. Inorg. Chem. 2012, 4945. DOI: 10.1002/ejic.201200620
- Wu. X.-F.; Neumann, H.; Beller, M. Chem. Eur. J. 2012, 7, 1744. DOI: 10.1002/asia.20120021
- Jin, Z.; Hammond, G. B.; Xu, B. Aldrichimica Acta 2012, 45, 67. [PDF]
-
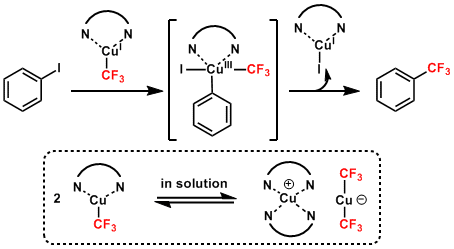
Reaction Mechanism
The active species is in equilibrium with the bis(trifluoromethyl)copper “ate” species. The exact mechanism is yet to be elucidated but the mechanism of the cross coupling is thought to be similar to those of other Cu-catalyzed couplings.
-
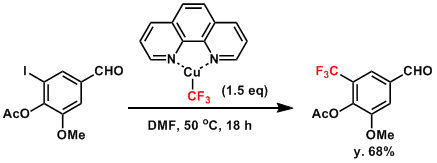
Examples
An example where CF3 group is introduced using TrifluoromethylatorTM.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem. Int. Ed. 2011, 50, 3793. DOI: 10.1002/anie.201100633
-
Related Reactions
Electrophilic Trifluoromethylation
Electrophilic Fluorination Reagent
-
Related Books
[amazonjs asin=”111807856X” locale=”US” title=”Efficient Preparations of Fluorine Compounds”]
[amazonjs asin=”0470193417″ locale=”US” title=”Guide to Fluorine NMR for Organic Chemists”]
[amazonjs asin=”B001DADOFY” locale=”US” title=”Bioorganic and Medicinal Chemistry of Fluorine”]
[amazonjs asin=”1848166346″ locale=”US” title=”Fluorine in Pharmaceutical and Medicinal ChemistryFrom Biophysical Aspects to Clinical Applications (Molecular Medicine and Medicinal Chemistry)”]
-
External Links