- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
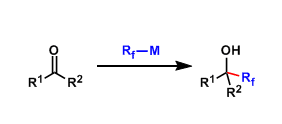
When one wants to introduce perfluoroalkyl groups onto carbonyl groups by nucleophilic addition, she/he needs fluorine-containing organometallic reagents. However, these reagents are not very stable and only few practical procedures are available. Even today, this is a developing area that has a room for further improvement.
The introduction of trifluoromethyl (CF3) group is the simplest. Typical conditions are Me3SiCF3 + F– (the Ruppert-Prakash reaction).
-
General References
<Ruppert-Prakash>
- Surya Prakash, G. K.; Krishmnamurti, R.; Olah, G. A. J. Am. Chem. Soc. 1989, 111, 393. DOI: 10.1021/ja00183a073
- Surya Prakash, G. K.; Panja, C.; Baghoo, H.; Surampudi, V.; Kultyshev, R.; Mandal, M.; Rasul, G.; Mathew, T.; Olah, G. A. J. Org. Chem. 2006,71, 6586. doi:10.1021/jo060835d
<review>
- Burton, D. J.; Yang, Z.-Y. Tetrahedron 1992, 48, 189. doi:10.1016/S0040-4020(01)88139-4
- McClinton, M. A.; McClinton, D. A. Tetrahedron 1992, 48, 6555. doi:10.1016/S0040-4020(01)80011-9
- Uno, H.; Suzuki, H. Synlett 1993, 91. DOI: 10.1055/s-1993-22361
- Surya Prakash, G. K.; Yudin, A. K. Chem. Rev. 1997, 97, 757. DOI: 10.1021/cr9408991
- Surya Prakash, G. K.; Mandal, M. J. Fluor. Chem. 2001, 112, 123. doi:10.1016/S0022-1139(01)00477-8
- Ma, J.-A.; Cahard, D. J. Fluor. Chem. 2007, 128, 975. doi:10.1016/j.jfluchem.2007.04.026
- Hu, J.; Zhang, W.; Wang, F. Chem. Commun. 2009, 7465. DOI: 10.1039/b916463d
- Dilman, A. D.; Levin, V. V. Eur. J. Org. Chem. 2011, 831. DOI: 10.1002/ejoc.201001558
-
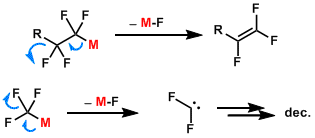
Reaction Mechanism
Fluorine-containing organometallic reagents are prone to decomposition through pathways such as these:
-
Examples
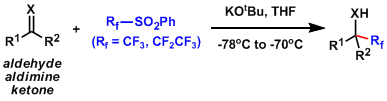
A pentafluoroethylating reagent that can be activated by alkoxide.[1]
The conditions involving in situ generation of perfluoroalkyltin (IV). The reagent is stable at room temperature and easily used. This procedure works well for fluoroalkylations other than trifluoromethylation.[2]
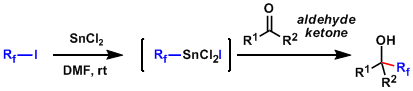
A newer system that uses DBU-hexafluoroacetone salt. The reagent is a solid that can be prepared on a large scale from inexpensive materials.[3]
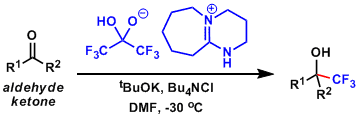
A borate-type trifluoromethylation reagent, which can be handled with ease.[4]
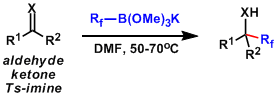
An interesting way to introduce difluoroester by Ru-catalyzed Reformatsky-type reaction.[5]
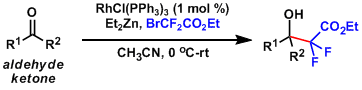
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Surya Prakash, G. K.; Wang, Y.; Mogi, R.; Hu, J.; Mathew, T.; Olah, G. A. Org. Lett. 2010, 12, 2932. doi:10.1021/ol100918d
[2] Kitazume, T.; Ishikawa, N. Chem. Lett. 1981, 1337. doi:10.1246/cl.1981.1337
[3] Riofski, M. V.; Hart, A. D.; Colby, D. A. Org. Lett. 2012, ASAP. doi:10.1021/ol303291x
[4] Levin, V. V.; Dilman, A. D.; Belyakov, P. A.; Struchkova, M. I.; Tartakovsky, V. A. Tetrahedron Lett. 2011, 52, 281. doi:10.1016/j.tetlet.2010.11.025
[5] Sato, K.; Tarui, A.; Kita, T.; Ishida, Y.; Tamura, H.; Omote, M.; Ando, A.; Kumadaki, I. Tetrahedron Lett. 2004, 45, 5735. doi:10.1016/j.tetlet.2004.05.099
-
Related Reactions
Electrophilic Trifluoromethylation
Electrophilic Fluorination Reagent
-
Related Books
[amazonjs asin=”0470021772″ locale=”US” title=”Fluorine-Containing Reagents (Hdbk of Reagents for Organic Synthesis)”]
[amazonjs asin=”111807856X” locale=”US” title=”Efficient Preparations of Fluorine Compounds”]
[amazonjs asin=”1848166346″ locale=”US” title=”Fluorine in Pharmaceutical and Medicinal ChemistryFrom Biophysical Aspects to Clinical Applications (Molecular Medicine and Medicinal Chemistry)”]
[amazonjs asin=”B001DADOFY” locale=”US” title=”Bioorganic and Medicinal Chemistry of Fluorine”]
-
External Links