- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
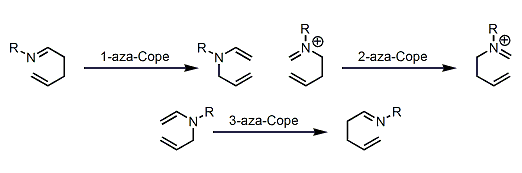
Nitrogen-containing 1,5-dienes undergo [3,3]-sigmatropic rearrangement just like the Cope rearrangement of 1,5-dienes. Among these so-called aza-Cope rearrangements, the 3-aza version is sometimes referred to as the aza-Claisen rearrangement. In well-designed systems, the 2-aza version is known to undergo the Mannich reaction sequentially, in which case the reaction is called the aza-Cope/Mannich reaction.
-
General References
- Horowitz, R. M.; Geissman, T. A. J. Am. Chem. Soc. 1950, 72, 1518 DOI: 10.1021/ja01160a025
Review
- Overman, L. E.; Humphreys, P. G.; Welmaker, G. S. Org. React. 2011, 75, 747. DOI:10.1002/0471264180.or075.04
-
Reaction Mechanism
Similar to other [3,3]-sigmatropic rearrangements, it is assumed that the reaction proceeds via a six-membered chair transition state (except for some specific cases in which it is not possible). The rearrangement is stereospecific for chiral compounds.
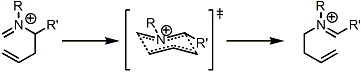
-
Examples
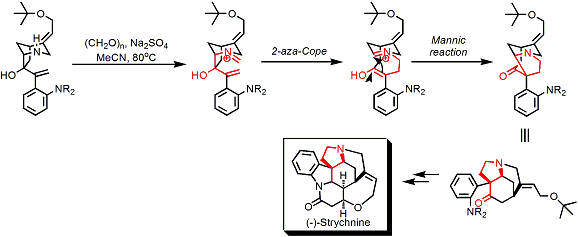
The iminium formed by the 2-aza-Cope rearrangement can be reacted with nucleophiles in the intramolecular Mannich fashion, which has been utilized in the synthesis of extremely complex alkaloid molecules.[1] Shown below is a famous example where it was used as a key step in the synthesis of (-)-strychnine.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Review: Overman, L. E. Acc. Chem. Res. 1992, 25, 352. DOI: 10.1021/ar00020a005
-
Related Reactions
- Ichikawa Allylcyanate Rearrangement
- Vinylcyclopropane Rearrangement
- Overman Rearrangement
- Eschenmoser-Claisen Rearrangement
- Oxy-Cope Rearrangement
- Cope Rearrangement
- Johnson-Claisen Rearrangement
- Ireland-Claisen Rearrangement
- Wittig Rearrangement
- Claisen Rearrangement
-
Related Books
-
External Links
Aza-Cope rearrangement – Wikipedia