- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
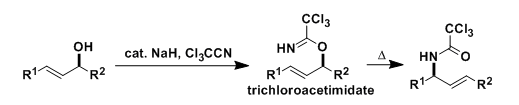
The trichloroacetimidates of allylic alcohols, prepared using trichloroacetonitrile, undergo thermal or catalyzed [3,3]-sigmatropic rearrangement, which is referred to as the Overman rearrangement. This reaction is an effective option to prepare allylic amines including synthetically challenging α-quaternary amines.
-
General References
- Overman, L. E. J. Am. Chem. Soc. 1974, 96, 597. DOI: 10.1021/ja00809a054
- Overman, L. E. J. Am. Chem. Soc. 1976, 98, 2901. DOI: 10.1021/ja00426a038
- Overman, L. E. Acc. Chem. Res. 1980, 13, 218. DOI: 10.1021/ar50151a005
- Nishikawa, T.; Asai, M.; Ohyabu, N.; Isobe, M. J. Org. Chem.1998, 63, 188. DOI: 10.1021/jo9713924
-
Reaction Mechanism
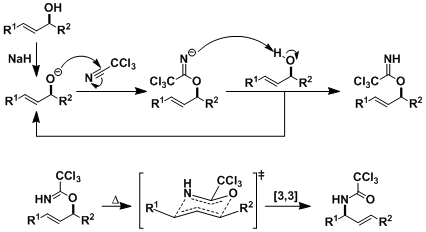
The rearrangement is believed to go through a six-membered chair transition state similar to the Claisen and other rearrangements.
-
Examples
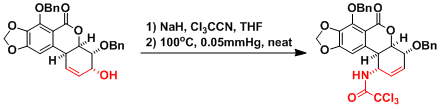
An application in the total synthesis of pancratistatin.[1]
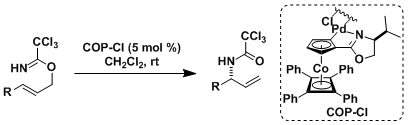
The COP (cobalt oxazoline palladacycle) catalyst was used to render the rearrangement enantioselective.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Danishefsky, S. J.; Lee, J. Y. J. Am. Chem. Soc. 1989, 111, 4829. DOI: 10.1021/ja00195a039
[2] (a) Anderson, C. E.; Overman, L. E. J. Am. Chem. Soc. 2003, 125, 12412. DOI: 10.1021/ja037086r
(b) Anderson, C. E.; Overman, L. E. Org. Synth. 2005, 82, 134. [website]
-
Related Reactions
- Ichikawa Allylcyanate Rearrangement
- Du Bois Amination
- Aza-Cope Rearrangement
- Eschenmoser-Claisen Rearrangement
- Oxy-Cope Rearrangement
- Cope Rearrangement
- Johnson-Claisen Rearrangement
- Ireland-Claisen Rearrangement
- Claisen Rearrangement
-
Related Books
[amazonjs asin=”3527308253″ locale=”US” title=”The Claisen Rearrangement: Methods and Applications”]
[amazonjs asin=”3527314393″ locale=”US” title=”Pericyclic Reactions – A Textbook: Reactions, Applications and Theory”]
-
External Links
- Overman Rearrangement (Wikipedia)
- Overman Rearrangement (organic-chemistry.org)