- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
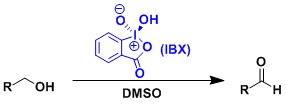
2-Iodoxybenzoic acid (IBX) is a mild and versatile oxidant. It can be easily prepared from 2-iodobenzoic acid and Oxone (See the Dess-Martin oxidation as reference).
IBX had long been rarely used as a reagent because of its poor solubility in organic solvents other than DMSO. New interesting reactions are being explored recently by Nicolaou and others.
-
General References
・Frigero, M.; Santagostino, M. Tetrahedron Lett. 1994, 35, 8019. DOI:10.1016/0040-4039(94)80038-3
・De Munari, S.; Frigero, M.; Santagostino, M. J. Org. Chem. 1996, 61, 9272. DOI: 10.1021/jo961044m
・Moore, J. D.; Finney, S. N. Org. Lett. 2002, 4, 3001. DOI: 10.1021/ol026427n
・Nicolaou, K. C.; Zhong, Y. L.; Baran, P. S. J. Am. Chem. Soc. 2000, 122, 7596. DOI: 10.1021/ja001825b
・Nicolaou, K. C.; Montagnon, T.; Baran, P. S.; Zhong, Y.-L. J. Am. Chem. Soc. 2002, 124, 2245. DOI:10.1021/ja012127+
・Nicolaou, K. C.; Mathison, C. J. N.; Montagnon, T. Angew. Chem. Int. Ed. 2003, 42, 4077. DOI:10.1002/anie.200352076
-
Reaction Mechanism
The addition of water is known to decrease the reactivity, which is rationalized by the reversible ligand exchange mechanism. (ref: J. Am. Chem. Soc. 2005, 127, 14146.)
-
Examples
Whereas the Dess-Martin reagent cleaves 1,2-diols, IBX can oxidize them without breaking them up.[1]
IBX is also useful to prepare α,β-unsaturated ketones.[2]
It can also oxidize benzylic methyl groups into aldehydes.[3]
The oxidation of amines to imines is also possible with IBX.[4]
The oxidation to carboxylic acids is possible by adding N-hydroxysuccinimide. The functional group selectivity is high.[5a] Using Oxone as a reoxidant under heating conditions, IBX can be substoichiometric.[5b]
Selective oxidation of secondary hydroxyl groups in the presence of primary hydroxyl groups is possible.[6]
2-Iodobenzenesulfonic acid acts as a highly active oxidation catalyst. It forms a sulfonic acid analogue of IBX in situ.[7]
The conversion of amides into nitriles with one-carbon dehomologation.[8]
-
Experimental Procedure
-
Experimental Tips
IBX has well-known for its hazard risks as a potential explosive.[9] Special caution is required when carrying out the reaction on a large scale.
-
References
[1]
[2]
[3]
[4]
[5] (a) Mazitschek, R.; Mulbaier, M. M.; Giannis, A. Angew. Chem. Int. Ed. 2002, 41, 4059. [abstract] (b) Thottumkara, A. P.; Bowsher, M. S.; Vinod, T. K. Org. Lett. 2005, 7, 2933. DOI: 10.1021/ol050875o
[6] Kuhakarn, C.; Kittigowittana, K.; Pohmakotr, M.; Reutrakul, V. Tetrahedron 2005, 61, 8995. DOI: 10.1016/j.tet.2005.07.051
[7] Uyanik, M.; Akakura, M.; Ishihara, K. J. Am. Chem. Soc. 2009, 131, 251. DOI: 10.1021/ja807110n
[8] Bhalarao, D. S; Mahajan, U. S.; Chaudhari, K. H.; Akamanchi, K. G. J. Org. Chem. 2007, 72, 662. DOI: 10.1021/jo0619074
[9] Plumb, J. B.; Harper, D. J. C&EN News 1990, July 16, 3.
-
Related Books
[amazonjs asin=”3642079067″ locale=”US” title=”Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis (Topics in Current Chemistry)”]
[amazonjs asin=”0123909481″ locale=”US” title=”Hypervalent Iodine in Organic Synthesis”]

![IBX_2[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_21.gif)
![IBX_3[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_31.gif)
![IBX_5[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_51.gif)
![IBX_4[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_41.gif)
![IBX_6[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_61.gif)
![IBX_7[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_71.gif)
![IBX_8[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_81.gif)
![IBX_9[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_91.gif)
![IBX_10[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_101.gif)
![IBX_11[1]](https://assets.en.chem-station.com/uploads/2014/04/IBX_111.gif)