- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
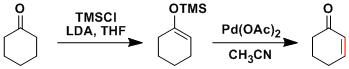
In the Saegusa-Ito reaction, silyl enol ethers are converted into corresponding α,β-unsaturated ketones using Pd(OAc)2.
There are modified versions developed to avoid the use of the expensive palladium reagent in stoichiometric amount. For example, there are a method that uses O2 as a reoxidant in DMSO (the Larock modification), a method that uses inexpensive Cu(II) as a reoxidant, and a method that uses allyl vinyl carbonates instead of silyl enol ethers (the Tsuji modification).
-
General References
・Ito, Y.; Hirao, T.; Saegusa, T. J. Org. Chem. 1978, 43, 1011. DOI:10.1021/jo00399a052
・Larock Modification: Larock, R. C.; Hightower, T. R.; Kraus, G. A.; Hahn, P.; Zheng, D. A. Tetrahedron Lett. 1995, 36, 2423. DOI:10.1016/0040-4039(95)00306-W
・Tsuji Modification: Shimizu, I.; Minami, I..; Tsuji, J. Tetrahedron Lett. 1983, 24, 1797. DOI:10.1016/S0040-4039(00)81773-6
-
Reaction Mechanism
-
Examples
An application in the total synthesis of norzoanthamine.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Miyashita, M.; Sasaki, M.; Hattori, I.; Sakai, M.; Tanino, K.Science 2004, 305, 495. DOI: 10.1126/science.1098851
-
Related Books
[amazonjs asin=”0471315060″ locale=”US” title=”Handbook of Organopalladium Chemistry for Organic Synthesis (2 Vol. Set)”]

![ito_saegusa_2[1]](https://assets.en.chem-station.com/uploads/2014/04/ito_saegusa_21.gif)
![ito_saegusa_3[1]](https://assets.en.chem-station.com/uploads/2014/04/ito_saegusa_31.gif)