- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-Upon heating, acyl azides undergo rearrangement to give isocyanates with the loss of N2. In the presence of water, the isocyanates are immediately hydrolyzed to give amines with one less carbon. The Curtius rearrangement is one of the few methods capable of synthesizing chiral amines in stereospecific manner.
-It is a synthetically flexible reaction that allows for the preparation of amines protected as carbamates (Boc, Cbz, etc.) by using appropriate alcohols instead of water.
-Sodium azide is potentially explosive. As an alternative way to prepare acyl azides, safer diphenylphosphorylazide (DPPA) is known. DPPA effects the conversion of carboxylic acids into acyl azides, then the rearrangement under mild conditions.
-
General References
・Curtius, T. Ber. 1890, 23, 3023.
・Curtius, T. J. Prakt. Chem. 1894, 50, 275.
・Shioiri, T.; Ninomiya, K.; Yamada, S.-i. J. Am. Chem. Soc. 1972, 94, 6203. DOI: 10.1021/ja00772a052
・Ninomiya, K.; Shioiri, T.; Yamada, S.-i. Tetrahedron 1974, 30, 2151. doi:10.1016/S0040-4020(01)97352-1
・Smith, P. A. S. Org. React. 1946, 3, 337.
・Scriven, E. F.; Turnbull, K.Chem. Rev. 1988, 88, 297. DOI: 10.1021/cr00084a001
・Wolff, O.; Waldvogel, S. R. Synthesis 2004, 1303. DOI: 10.1055/s-2004-815965
-
Reaction Mechanism
The rearrangement proceeds through the acyl nitrene-like intermediate and with the retention of stereochemistry. When R’=H (water), the ensuing decarboxylation leads to the formation of an amine product.
-
Examples
An application of DPPA (aka. the Shioiri reagent).[1]
A example showing the powerful utility to construct an α-quaternary amine.[2]
An application in the synthesis of Tamiflu.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Zhang, Q. et al. J. Org. Chem. 2000, 65, 7977. DOI: 10.1021/jo000978e
[2] Murashige, K. et al. Tetrahedron 2002, 58, 4917. doi:10.1016/S0040-4020(02)00436-2
[3] Yamatsugu, K.; Yin, L.; Kamijo, S.; Kimura, Y.; Kanai, M.; Shibasaki, M. Angew. Chem., Intl. Ed. 2009, 48, 1070. DOI: 10.1002/anie.200804777
-
Related Books
[amazonjs asin=”0198556721″ locale=”US” title=”Reactive Intermediates (Oxford Chemistry Primers)”]

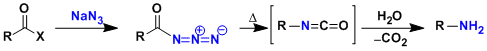
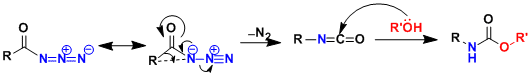
![curtiu2[1]](https://assets.en.chem-station.com/uploads/2014/03/curtiu21.gif)
![curtius_4[1]](https://assets.en.chem-station.com/uploads/2014/03/curtius_41.gif)