- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
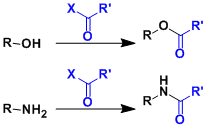
-Acyl groups are generally stable under acidic and oxidative conditions and deprotected under reductive (DIBAL, LAH, etc.) and basic (NaOH, K2CO3/MeOH etc.) conditions.
-Acetyl, pivaloyl, and benzoyl groups are typical examples. Pivaloyl protection can be done selectively for sterically uncongested hydroxyl groups.
-Amines can be protected as amides, but deprotection usually requires more forcing conditions (strongly acidic or basic at high temperatures). Except for relatively labile ones such as trifluoroacetyl group, acyl groups are used less commonly for the protection of amines than carbamate groups.
-One should be careful that substrates containing multiple hydroxyl groups are potentially prone to acyl migration.
-
General References
-
Reaction Mechanism
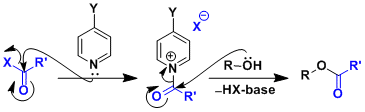
Pyridine bases are generally used. The mechanism involves the generation of a reactive acyl pyridinium species.
4-Dimethylaminopyridine (DMAP) in particular is known as an effective nucleophilic catalyst. Capable of increasing the reaction rate up to 10,000 times (with acetic anhydride as the reagent), DMAP allows for the protection of unreactive alcohols. (Ref: Angew. Chem., Int. Ed. Engl. 1978, 17, 522.)
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books
[amazonjs asin=”0471697540″ locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”]