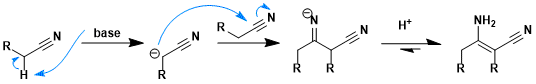- Popularity
- Criteria #2
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
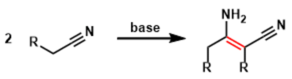
The reaction between a nitrile and an alkoxide base leads to the formation of a β-iminonitrile from which an α-cyanoketone is obtained by mild acid hydrolysis. This reaction is known as the Thorpe reaction when intermolecular, and the Thorpe-Ziegler reaction when intramolecular. It is to say that this reaction is a series of Claisen condensation.
The Thorpe-Ziegler reaction is especially useful in the formation of five- to eight membered rings and for rings with more than thirteen members, although it fails for nine- to twelve-membered rings.
-
Literature reference
・ Baron, H.; Remfry, F. G. P.; Thorpe, Y. F. J. Chem. Soc. 1904, 85, 1726.
・ Karl Ziegler et al. Ann. 1933, 504, 94.
・ Schaefer, J. P.; Bloomfield, J. J. Org. React. 1967, 15, 1.
-
Reaction mechanism
-
Related Books
[amazonjs asin=”1166418006″ locale=”US” title=”A Study Of The Claisen Condensation (1908)”]
-
Related Links
・Thorpe Reaction - Wikipedia