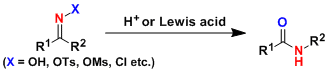- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-The Beckmann rearrangement is the acid-induced rearrangement of oximes or its derivatives, which after hydration and tautomerization produces amides. Cyclic oximes give ring-expanded amides.
-The Beckmann rearrangement of cyclohexaneoxime is used in industry to synthesize ε-caprolactam, an important ingredient of 6-nylon.
-
General References
・ Beckmann, E. Ber. 1886, 19, 988.
・ Donaruma, L. G.; Heldt, W. Z.; Org. React. 1960, 11, 1.
・ Gawtey, R. E. Org. React. 1988, 35, 1.
・ Hauske, J. R. Comp. Org. Syn. 1991, 1, 98.
・ Maruoka, K.; Yamamoto, H. Comp. Org. Syn. 1991, 6, 763.
・ Craig, D. Comp. Org. Syn. 1991, 7, 689.
-
Reaction Mechanism
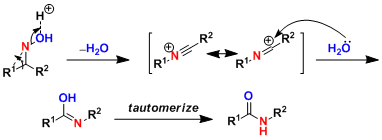
-Rearrangement from the side trans to the OH group is favored based on molecular orbital consideration. However, the E/Z isomerization of oxime is fast under acidic conditions, thus the regioselectivity is determined by the functional groups on both sides. The order of relative tendency to rearrange is aryl, alkenyl > 3˚alkyl > 2˚alkyl > 1˚alkyl, which is correlated with electron richness. (Ref: Angew. Chem. Int. Ed. 2005, 44, 2370.)
-
Examples
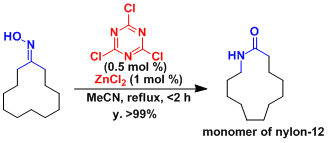
Yamamoto and Ishihara developed the catalytic system consisting of cyanuric chloride and zinc chloride.[1] Their proposed mechanism involving a Meisenheimer complex is interesting.
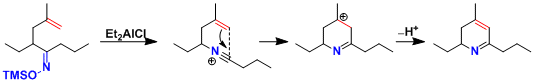
Dihydropyridines can be synthesized by trapping the intermediate intramolecularly with a C-nucleophile.[2]
The synthesis of pinnaic acid[3]: The rearrangement using the MSH reagent[4] is used to construct the α-quarternary amine.
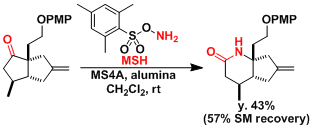
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Furuya, Y.; Ishihara, K.; Yamamoto, H. J. Am. Chem. Soc. 2005, 127, 11240. DOI: 10.1021/ja053441x
[2] 大学院講義有機化学 II, p138.
[3] Xu, S.; Arimoto, H.; Uemura, D. Angew. Chem. Int. Ed. 2007, 46, 5764. DOI:10.1002/anie.200701581
[4] Tamura, Y.; Fujiwara, H.; Sumoto, K.; Ikeda, M.; Kita, Y. Synthesis 1973, 215.
[5] Schinzer, D.; Bo, Y. Angew. Chem. Int. Ed. 1991, 30, 687. DOI: 10.1002/anie.199106871
-
Related Books