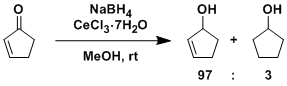- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-While 1,4-reduction usually predominates in the reduction of α,β-unsaturated ketones with NaBH4 alone, selective 1,2-reduction is possible using CeCl3-7H2O as an additive. Luche reduction can be used for a wide range of compounds and reaction times are also short (3-5 min). Simple procedure is another plus for this reaction. DIBAL is used for the same purpose but it requires lower temperature.
-
General References
・ Luche, J. L. J. Am. Chem. Soc. 1978, 100, 2226. DOI: 10.1021/ja00475a040
・ Gemal, A. L.; Luche, J. L. J. Am. Chem. Soc. 1981, 103, 5454. DOI: 10.1021/ja00408a029
-
Reaction Mechanism
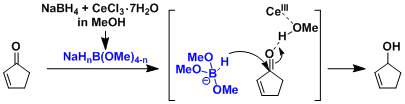
Aside from acting as a hard Lewis acid, cerium is considered to promote ligand exchange on the boron atom leading to the formation of NaHnB(OMe)4-n, a harder reducing agent than NaBH4.
-
Examples
In the substrate containing both ketone and aldehyde groups, Ce(III)-catalyzed acetalization (or hemiacetalization or hydration) prevents the aldehyde from getting reduced, enabling selective reduction of the ketone.
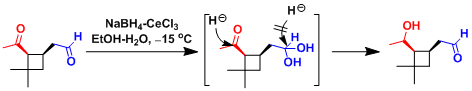
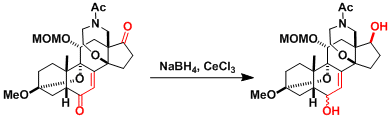
An example in the synthesis of batracotoxinin.[1]
-
Experimetal Procedure
-
Experimental Tips
-
References
[1] Kurosu, M.; Marcin, L. R.; Grinsteiner, T. J.; Kishi, Y. J. Am. Chem. Soc. 1998, 120, 6627. doi: 10.1021/ja981258g
-
Related Books