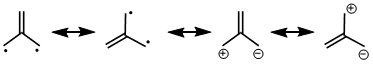- Reliability
- Popularity
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
Trimethylenemethane (TMM) [3 + 2] cycloadditions provide direct routes to functionalized cyclopentanes. This reaction has been shown to be a highly chemo-, regio-, and diastereoselective process.
In considering a zwitterion equivalent of TMM, synthons for a carbanion and carbocation that do not self-annihilate are required. Silyl and stannyl groups serve as carbanion equivalents; carboxylate as well as more reactive leaving groups such as methanesulfonate and halide may serve as carbocation equivalents.
-
Literature reference
・Baseman, R. J.; Pratt, D. W.; Chow, M.; Dowd, P. J. Am. Chem. Soc. 1976, 98, 5726. DOI:10.1021/ja00434a068
・Trost, B. M.; Chan, D. M. T. J. Am. Chem. Soc. 1979, 101, 6429. DOI:10.1021/ja00515a046
・Yamago, S.; Nakamura, E. J. Am. Chem. Soc. 1989, 111, 7285. DOI: 10.1021/ja00200a072
・ Nakamura, E.; Yamago, S.; Ejiri, S.; Dorigo, A. E.; Morokuma, K. J. Am. Chem. Soc. 1991, 113, 3183. DOI: 10.1021/ja00008a063
<review>
・Huisgen, R. Angew. Chem. Int. Ed. 1963, 2, 565. DOI:10.1002/anie.196305651
・Huisgen, R. Angew. Chem. Int. Ed. 1963, 2, 633. DOI:10.1002/anie.196306331
・Dowd, P. Acc. Chem. Res. 1972, 5, 242. DOI: 10.1021/ar50055a003
・Berson, J. A. Acc. Chem. Res. 1978, 11, 446. DOI: 10.1021/ar50132a003
・Trost, B. M. Angew. Chem. Int. Ed. Engl. 1986, 25, 1. DOI: 10.1002/anie.198600013
・Trost, B. M. Pure Appl. Chem. 1988, 60, 1615. doi:10.1351/pac198860111615
・Nakamura, E.; Yamago, S. Acc. Chem. Res. 2002, 35, 867. DOI: 10.1021/ar0100935
・Yamago, S.; Nakamura, E. Org. React. 2003, 61, 1. DOI:10.1002/0471264180.or061.01
-
Reaction mechanism
The reactions are divided into five categories, defined by reaction types and precursors: (1) cycloadditions of free TMMs generated from diazenes, (2) cycloadditions of free TMMs generated from MCPs, (3) transition metal catalyzed reactions of MCPs, (4) transition metal catalyzed reactions of silylated allylic acetates, and (5) cycloaddition of stable TMM-metal complexes.
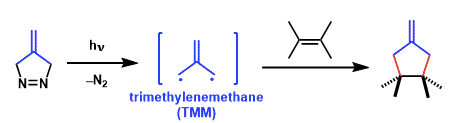
(1) cycloadditions of free TMMs generated from diazenes
The isopropylidene diyls are generated either thermally or photochemically from bicyclic azo compounds such as diazenes.
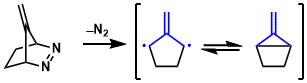
(2) cycloadditions of free TMMs generated from MCPs
When stabilizing groups are present, methylenecyclopropenes (MCPs) may open to the corresponding zwitterionic TMMs. Acetal has been used in this context, and provides cyclopentanes with the acetal functionality exo to the newly formed ring with high selectivity.
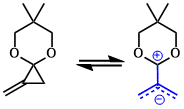
(3) transition metal catalyzed reactions of MCPs
(4) transition metal catalyzed reactions of silylated allylic acetates
Silylated allylic acetates, carbonates and other substituted allyl compounds may form TMM synthons under palladium catalysis. The reaction is highly regioselective, providing only the substitution pattern shown below regardless of the position of the R’ group on the starting allylic acetate.
(5) cycloaddition of stable TMM-metal complexes.
-
Example of reactions
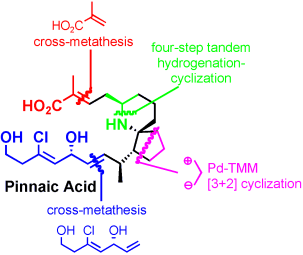
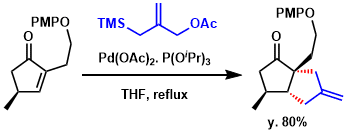
In the total synthesis of Pinnaic Acid, Pd-catalyzed trimethylenemethane [3+2] cyclization (Pd-TMM cyclization) was employed. Pd-TMM [3+2] cyclization of a cyclopentanone derivative proceeded with high stereoselectivity and only the anti adduct could be isolated (80% yield).[1]
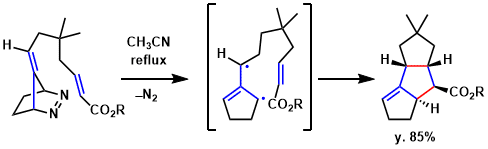
In the total synthesis of Hirsutene, a regiospecific and highly stereoselective intramolecular diyl trapping reaction was conducted to construct the requisite linearly fused tricyclopentanoid ring system.[2]
Using an intramolecular diyl trapping route to synthesize linearly fused tricyclopentanoids could be tested, azo compound was refluxed in acetonitrile for 6 h. A highly stereoselective reaction ensued; after removal of the solvent and chromatographic purification, tricyclopentanoid was isolated in 85% yield.
-
Bibliography
[1] “Asymmetric Total Synthesis of Pinnaic Acid”
Xu, S.; Arimoto, H.; Uemura, D. Angew. Chem. Int. Ed. 2007, 46, 5746. DOI:10.1002/anie.200701581
Pinned together: Asymmetric total synthesis of pinnaic acid was accomplished through a stereospecific route that features as key steps a Pd-catalyzed trimethylenemethane (TMM) [3+2] cyclization, a four-step tandem hydrogenation–cyclization, and cross-olefin-metathesis reactions (see scheme).
[2] Little, R. D.; Muller, G. W. J. Am. Chem. Soc. 1981, 103, 2744. DOI: 10.1021/ja00400a043
-
Related Books
[amazonjs asin=”1118299884″ locale=”US” title=”Methods and Applications of Cycloaddition Reactions in Organic Syntheses”][amazonjs asin=”3846536490″ locale=”US” title=”Azomethines and 1,3 Dipoles – Leading to New Heterocycles: Studies on 1,3 Dipolar Cycloadditions”]
-
Related Links
・1,3-Dipole (Wikipedia) ・1,3-Dipolar cycloaddition (Wikipedia) ・Trimethylenemethane - Wikipedia ・Trimethylenemethane Cycloaddition - Wikipedia ・Asymmetric Palladium-Catalyzed [3+2] Cycloadditions of Trimethylenemethane (PDF)