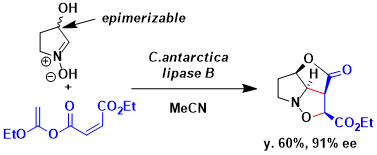- Popularity
- Stereoselectivity
- Atom efficiency
- Criteria #4
- Criteria #5
-
Characteristics
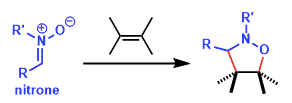
The 1,3-dipolar cycloaddition of nitrones, a kind of 1,3-dipole to unsaturated (C–C double or triple bond) compounds is one of the useful synthetic method for preparing isoxazole, dihydro- and tetrahedron-isoxazole derivatives. Particularly, the nitrone–olefin 1,3-dipolar cycloaddition is a powerful reaction in that it can create as many as three new contiguous stereogenic centers in a single step.
-
Literature reference
<review>
・Huisgen, R. Angew. Chem. Int. Ed. 1963, 2, 565. DOI:10.1002/anie.196305651
・Huisgen, R. Angew. Chem. Int. Ed. 1963, 2, 633. DOI:10.1002/anie.196306331
・Confalone, P. N.; Huie, E. M. Org. React. 1988, 36, 1. DOI:10.1002/0471264180.or036.01
・Gothelf, K. V.; Jorgensen, K. A. Chem. Rev. 1998, 98, 863. DOI: 10.1021/cr970324e
・Pellissier, H. Tetrahedron 2007, 63, 3235. DOI:10.1016/j.tet.2007.01.009
-
Reaction mechanism
See, 1,3-dipolar cycloaddition (Not prepared yet)
-
Example of reactions
Kita and coworkers reported lipase-catalyzed domino kinetic resolution of alpha-hydroxynitrones/intramolecular 1,3-dipolar cycloaddition for the asymmetric total synthesis of (–)-rosmarinecine.[1] A domino reaction of the cyclic nitrone with the acyl donors in the presence of Candida antarctica lipase, fraction B underwent the kinetic resolution of cyclic nitrone, and subsequent intramolecular 1,3-dipolar cycloaddition to gave the corresponding products as single enantio- and diasteromer (91% ee).
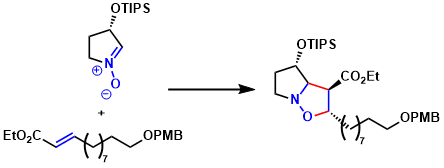
Nagasawa and coworkers reported an enantioselective total synthesis of batzelladine A based upon a strategy involving successive 1,3-dipolar cycloadditions.[2] The 1,3-diploar cycloaddition reaction between 10 and the ester proceeded stereoselectively to give the isoxazolidine 12 as a single diastereomer.
-
Bibliography
[1] Akai, S.; Tanimoto, K.; Kanao, Y.; Omura, S.; Kita, Y. Chem. Commun. 2005, 2369. DOI:10.1039/B419426H
[2] Shimokawa, J.; Shirai, K.; Tanatani, A.; Hashimoto, Y.; Nagasawa, K. Angew. Chem. Int. Ed. 2004,43. 1559. DOI: 10.1002/anie.200353200
-
Related Books
[amazonjs asin=”3527314393″ locale=”US” title=”Pericyclic Reactions – A Textbook: Reactions, Applications and Theory”][amazonjs asin=”3527320881″ locale=”US” title=”Handbook of Cyclization Reactions”]
-
Related Links
・1,3-Dipole (Wikipedia) ・1,3-Dipolar cycloaddition (Wikipedia) ・Nitrone - Wikipedia ・nitone-olefin 3+2 cycloaddition - Wikipedia ・Catalysis of 1,3-Dipolar Cycloaddition of Nitornes (Denmark Group, PDF) ・1,3-Dipolar Cycloaddition Reactions of Nitrones with Alkenes (Takeda Group, PDF)