- Popularity
- Criteria #2
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
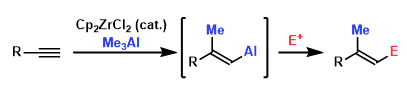
Ngishi carbometalation is shown to involve one-step syn-addition of a Me–Zr bond to terminal alkynes in an anti-Markovnikov manner followed by Zr-to-Al transmetalation on the resultant carbon group.
Zr-catalyzed regio- and stereoselective methylalumination across the terminal alkene as precursor in the presence of a stoichiometric alkylalminum reagent, and a zirconium catalyst s first step. The formed alkenylaluminum compound is transmetalated to zinc to afford the corresponding alkenylzinc compound, which reacts consecutively with electrophiles leading to the substituted alkenes.
This method is vey advantageous from the viewpoint of the facile formation of the organizing reagent without the addition of the bases. Additionally , the alkenylaluminum intermediate can be used for Negishi coupling sequentially.
-
Literature reference
・Van Horn, D. E.; Negishi, E. J. Am. Chem. Soc. 1978, 100, 2252. DOI: 10.1021/ja00475a058
<review>
・Negishi, E. Bull. Chem. Soc. Jpn. 2007, 80, 233. DOI:10.1246/bcsj.80.233
・Negishi, E.; Wang, G.; Rao, H.; Xu, Z. J. Org. Chem. 2010, 75, 3151. DOI:10.1021/jo1003218
-
Related Books
[amazonjs asin=”0471315060″ locale=”US” title=”Handbook of Organopalladium Chemistry for Organic Synthesis (2 Vol. Set)”]