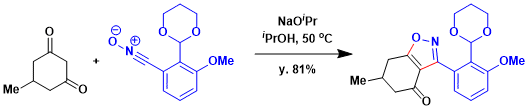- Popularity
- User-friendly
- Reliability
- Criteria #4
- Criteria #5
-
Characteristics
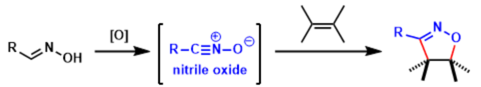
Nitrile oxides, R-C≡N+-O- are organic compounds which contain a monovalent functional group -CNO, which bound directly to a carbon atom of the organic moiety of a molecule. Most nitrile oxides are highly reactive and in the absence of trapping agents they undergo rapid dipolar cycloaddition with themselves to give furoxans. 1,3-Dipolar cycloaddition of nitrile oxide to C=C bond of dipolarophile is of considerable importance in organic synthesis, since this reaction yields 2-isoxazolines. Isoxazole and isoxazolines act as versatile building blocks. In General, the reactivity of nitrile oxides is higher than nitrones.
-
Literature reference
・Mukaiyama, T.; Hoshino, T. J. Am. Chem. Soc. 1960, 82, 5339. DOI: 10.1021/ja01505a017
・Morrochi, S.; Ricca, A.; Zanarotti, A. Tetrahedron Lett. 1969, 10, 3329. DOI:10.1016/S0040-4039(00)99754-5
・Shimizu, T.; Hayashi, Y.; Shibafuchi, H.; Teramura, K. Bull. Chem. Soc. Jpn. 1986, 59, 2827. DOI:10.1246/bcsj.59.2827
・Basel, Y.; Hassner, A. Syhthesis 1997, 309. DOI: 10.1055/s-1997-1181
<review>
・Huisgen, R. Angew. Chem. Int. Ed. 1963, 2, 565. DOI:10.1002/anie.196305651
・Huisgen, R. Angew. Chem. Int. Ed. 1963, 2, 633. DOI:10.1002/anie.196306331
・Grundmann, C. Synthesis 1970, 344. DOI: 10.1055/s-1970-21611
-
Reaction mechanism
See, 1,3-dipolar cycloaddition (Not prepared yet)
-
Example of reactions
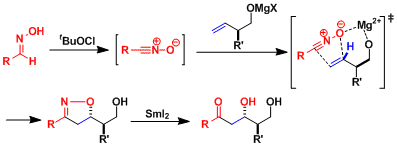
Carreira and coworkers reported Stereoselective syntheses of epothilones A and B via directed nitrile oxide cycloaddition.[1a] The application of a directed nitrile oxide cycloaddition was inspired by the work of Kanemasa,[1b]who has reported the diastereoselective cycloaddition reaction of simple aromatic nitrile oxides and allylic alcohols. Oxidation of the oxime to the nitrile oxide was followed by highly diastereoselective cycloaddition with commerically available (R)-3-butene-2-ol to furnish isoxazoline in 79% yield as a single diastereomer. Thereafter, treatment of isoxazoline with SmI2 (THF, 0 °C) resulted in clean reduction of the N–O bond.
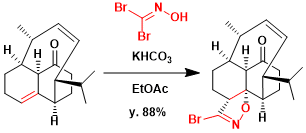
Baran and coworkers demonstrated 1,3-diploar cycloaddition of nitrile oxide to install the C8-methyl and C8a-hydroxy group on the synthesis of vinigrol.[2] Exposure of olefin to bromonitrile oxide (generated in situ from dibromoformaldoxime and KHCO3) resulted in a dipolar cycloaddition, leading to the formation of bromoisoxazoline as a single isomer in 88% yield on a gram scale. This cycloaddition proceeds with complete control over regio- and positional selectivity to produce a single diastereomer of bromoisoxazoline.
Suzuki and coworkers utilized a 1,3-dipolar cycloaddition of nitrile oxides leading to the coleophomone natural products.[3] Transformation to stable nitrile oxide, cyclocondensation with 5- methylcyclohexan-1,3-dione in the presence of sodium isopropoxide provided key isoxazole in 81% yield.
-
Bibliography
[1] (a) Bode, J. W.; Carreira, E. M. J. Am. Chem. Soc. 2001, 123, 3611. DOI: 10.1021/ja0155635 (b) Kanemasa, S.; Nishiuchi, M.; Kamimure, A.; Hori, K. J. Am. Chem. Soc. 1994, 116, 2324. DOI: 10.1021/ja00085a012 (c) Curran, D. P. J. Am. Chem. Soc. 1982, 104, 4024. DOI: 10.1021/ja00378a050
[2] Maimone, T.; Shi, J.; Ashida, S.; Baran, P. S. J. Am. Chem. Soc. 2009, 131, 17066. DOI:10.1021/ja908194b
[3] Bode, J. W.; Suzuki, K. Tetrahedron Lett. 2003, 44, 3559. doi:10.1016/S0040-4039(03)00615-4
-
Related Books
[amazonjs asin=”3527314393″ locale=”US” title=”Pericyclic Reactions – A Textbook: Reactions, Applications and Theory”][amazonjs asin=”3527320881″ locale=”US” title=”Handbook of Cyclization Reactions”]
-
Related Links
・1,3-Dipole (Wikipedia) ・1,3-Dipolar cycloaddition (Wikipedia) ・Nitrile Oxide Cycloadditions in Organic Synthesis (PDF)