- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
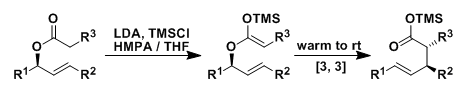
The Ireland-Claisen rearrangement is a version of the Claisen rearrangement in which ketene silyl acetals (prepared from allyl esters) undergo [3,3]-sigmatropic rearrangement to produce γ,δ-unsaturated carboxylic acids.
The synthetic importance of this reaction comes from its versatile and reliable stereocontrol, which is possible because ketene silyl acetals can be prepared in either E or Z geometry and the rearrangement proceeds at relatively low temperatures.
Other variations of the Claisen rearrangement involving allyl ester enolates include the use of zinc enolates (the Reformatsky-Claisen rearrangement) and acetoacetic acid ester enolates (the Carroll rearrangement).
The preparation of ketene silyl acetals requires strong bases. In cases when one needs to avoid basic conditions, analogous rearrangements are available under acidic conditions (the Johnson-Claisen rearrangement) and neutral conditions (the Eschenmoser-Claisen rearrangement).
-
General References
- Ireland, R. E.; Mueller, R. H. J. Am. Chem. Soc. 1972, 94, 5897. doi:10.1021/ja00771a062
- Ireland, R. E.; Willard, A. K.Tetrahedron Lett. 1975, 16, 3975. doi:10.1016/S0040-4039(00)91213-9
- Ireland, R. E. J. Am. Chem. Soc. 1976, 98, 2868. doi:10.1021/ja00426a03
- Ireland, R. E.; Wipf, P.; Armstrong, J. D., III J. Org. Chem. 1991, 56, 650. DOI: 10.1021/jo00002a030
- Ireland, R. E.; Wipf, P.; Xiang, J. N. J. Org. Chem. 1991, 56, 3572. DOI: 10.1021/jo00011a023
- Pereira, S.; Srebnik, M. Aldrichimica Acta 1993, 26, 17. [PDF]
- Hattori, K.; Yamamoto, H, Tetrahedron 1994, 50, 3099. doi:10.1016/S0040-4020(01)81109-1
- Chai, Y.; Hong, S.; Lindsay, H. A.; McFarland, C.; McIntosh, M. C. Tetrahedron 2002, 58, 2905. doi:10.1016/S0040-4020(02)00164-3
- Castro, A. M. M. Chem. Rev. 2004, 104, 2939. DOI: 10.1021/cr020703u
-
Reaction Mechanism
In the deprotonation of allyl esters by LDA, Z-enolates are formed in the absence of HMPA and E-enolates are formed in the presence of it. Both isomers of ketene silyl acetals can be prepared selectively in this way.
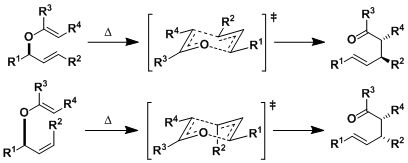
The ketene silyl acetal undergoes concerted [3,3]-sigmatropic rearrangement through a six-membered chair transition state. As is the case in the Claisen and other rearrangements, the reaction is generally stereospecific.
-
Examples
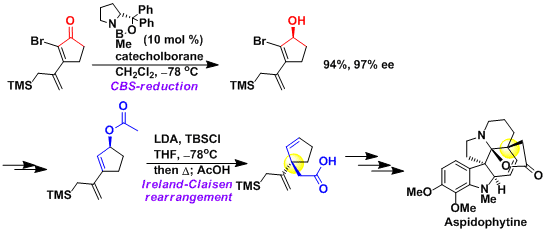
The Claisen rearrangement is very useful when constructing quaternary stereogenic carbons, since chiral allyl alcohols (the starting materials) can be accessed relatively easily. In the example shown here, the chirality introduced by the CBS reduction was transferred nicely to make one of the all-carbon quaternary stereogenic centers of aspidophytine.[1]
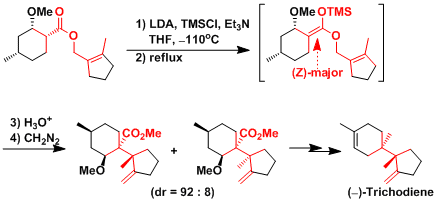
Even compounds containing contiguous quaternary stereogenic carbons, such as (-)-trichodiene, can be synthesized.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Corey, E. J. et al. J. Am. Chem. Soc. 1999, 121, 6771. DOI: 10.1021/ja9915201 [2] Gilbert, J. C. et al. J. Org. Chem. 1993, 58, 6255. DOI: 10.1021/jo00075a020
-
Related Reactions
- Ichikawa Allylcyanate Rearrangement
- Overman Rearrangement
- Aza-Cope Rearrangement
- Eschenmoser-Claisen Rearrangement
- Oxy-Cope Rearrangement
- Cope Rearrangement
- Johnson-Claisen Rearrangement
- Claisen Rearrangement
-
Related Books
[amazonjs asin=”3527308253″ locale=”US” title=”The Claisen Rearrangement: Methods and Applications”]
-
External Links