- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
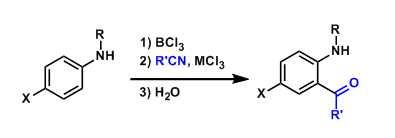
The ortho-acylation of unprotected anilines using nitriles is known as the Sugasawa reaction.
The Friedel-Crafts acylation of anilines is inherently difficult because the amino group coordinates with the Lewis acid and the resulting aromatic system is deactivated. The Sugasawa reaction is unique in that it uses two Lewis acids within one system.
The combination of BCl3-AlCl3 is employed most commonly, but the use of GaCl3 (which has a higher affinity for chlorine) in place of AlCl3 sometimes allows the reaction to proceed under milder conditions.
-
General References
・Sugasawa, T.; Toyoda, T.; Adachi, M.; Sasakura, K. J. Am. Chem. Soc. 1978, 100, 4842. DOI: 10.1021/ja00483a034
・Douglas, A. W.; Abramson, N. L.; Houpis, I. N.; Karady, S.; Molina, A.; Xavier, L. C.; Yasuda, N. Tetrahedron Lett. 1994, 35, 6807. doi:10.1016/0040-4039(94)85010-0
・Prasad, K.; Lee, G. T.; Chaudhary, A.; Girgis, M. J.; Streemke, J. W.; Repic, O. Org. Proc. Res. Dev. 2003, 7, 723. DOI: 10.1021/op0340659
-
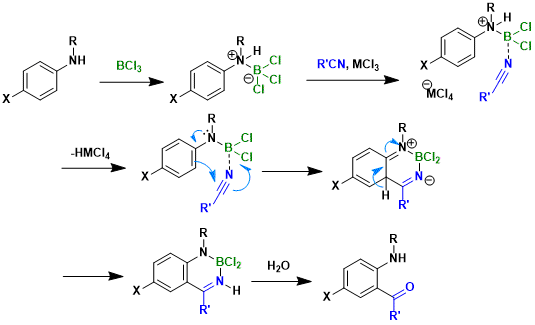
Reaction Mechanism
Ref: Tetrahedron Lett.1994, 35, 6807.
-
Examples
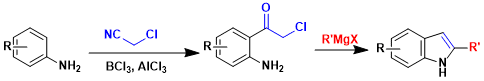
In this example, ortho-acylated anilines (prepared by the Sugasawa reaction) are carried on to synthesize substituted indoles.
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Pei, T.; Tellers, D. M.; Streckfuss, E. C.; Chen, C.-y.; Davies, I. W. Tetrahedron 2009, 65, 3285. doi:10.1016/j.tet.2008.11.026
-
Related Books
[amazonjs asin=”3527324704″ locale=”US” title=”The Art of Process Chemistry”]
-
External Links