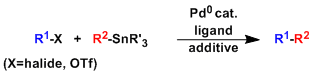- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-The palladium-catalyzed cross coupling of organic halides or triflates with organotin compounds is known as the Migita-Kosugi-Stille coupling.
-The reaction works under very mild (almost neutral) conditions and is therefore used frequently in the late stages of natural product and complex molecule synthesis.
-The biggest downside of this reaction is the chronic toxicity of organic tin compounds used in stoichiometric amounts. Special caution is required when the product of this reaction is to be used for bioassay.
-
General References
・ Kosugi, M. Sasazawa, K.; Shimizu, Y.; Migita, T. Chem. Lett. 1977, 301. doi:10.1246/cl.1977.301
・ Milstein, D.; Stille, J. K. J. Am. Chem. Soc. 1978, 100, 3636. doi:10.1021/ja00479a077
・ Stille, J. K. Angew. Chem. Int. Ed. Engl. 1986, 25, 508. doi:10.1002/anie.198605081
・ Farina, V.; Krishnamurthy, V.; Scott, W. J. Org. React. 1997, 50, 1.
・ Mitchell, T. N. Synthesis 1992, 803. doi:10.1055/s-1992-26230
・ Review for Pd-Catalyzed Cross Coupling in Total Synthesis: Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem. Int. Ed. 2005, 44, 4442. doi:10.1002/anie.200500368
-
Reaction Mechanism
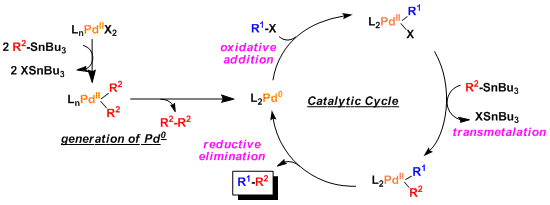
-The general catalytic cycle is similar to that of other palladium catalyzed coupling reactions.
–Normally, transmetallation is the rate determining step. The accelerative effect of additives is thought to be due to the promotion of the transmetallation process. (Ref: Angew. Chem. Int. Ed. 2004, 43, 4704.)
-The order of the rate of transmetallation from tin is: alkynyl > alkenyl > aryl > allyl, benzyl > α-alkoxyalkyl > alkyl. Therefore, the butyl and methyl groups from the organotin reagents rarely participate in the reaction.
-
Examples
The recent progress of cross coupling chemistry has been remarkable. Less reactive aryl chlorides can now be used as viable substrates.[1]

An example showing the effect of LiCl as an additive.[2]
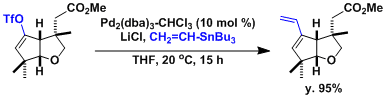
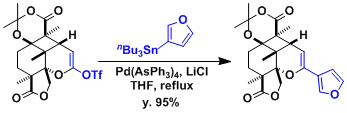
An example in the context of the synthesis of saudin.[3]
-
Experimental Procedure
-
Experimental Tips
-The organic tin residue tends to tail in silica gel columns and is difficult to clean. Its removal can be made somewhat easier by converting it to tin fluorides by stirring it in an aqueous KF solution overnight.
-The addition of LiCl is known to accelerate the reaction, the rationale of which is that the strong Sn-Cl interaction promotes transmetallation.
-The presence of excess ligand interferes with transmetallation. As a ligand scavenger, CuI is sometimes added for improvement. It is also known that ligands having moderate electron richness such as triphenylarsine (Ph3As) sometimes give good results.
-Fluoride sources like CsF are also known to promote the reaction, as it is suggested that they react with the organotin to form insoluble tin fluoride, which is expelled from the system.
-
References
[1] Littke, A. F.; Fu, G. C.Angew. Chem. Int. Ed. 1999, 38, 2411. [abstract]
[2] Piers, E. et al. Tetrahedron 1991, 47, 4555. doi:10.1016/S0040-4020(01)86462-0
[3] Winkler, J. D.; Doherty, E. M. J. Am. Chem. Soc. 1999, 121, 7425. DOI: 10.1021/ja9916198
-
Related Books
[amazonjs asin=”3527305181″ locale=”US” title=”Metal-Catalyzed Cross-Coupling Reactions (2 Volume Set)”]
[amazonjs asin=”3540421750″ locale=”US” title=”Cross-Coupling Reactions: A Practical Guide (Topics in Current Chemistry)”]
[amazonjs asin=”0471312738″ locale=”US” title=”The Stille Reaction”]