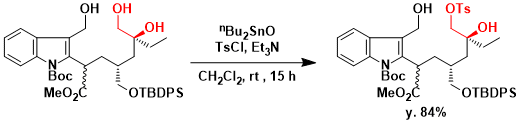- Reliability
- Versatility
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
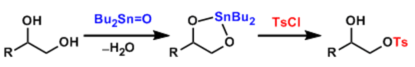
Typically, the 1,2-diol is treated with Bu2SnX, where X = O or (OMe), with removal (azeotropic or desiccant) of either H2O or MeOH to afford the requisite stannylidenes (“tin acetal”)
The stannylidenes undergo selective alkylation, acylation, sulfonylation, and phosphorylation, usually at the primary position, or silylation with variable regioselectivity. The tin acetal protocol accomplishes primary hydroxyl activation and temporary secondary hydroxyl protection in a single operation.
-
Literature reference
・Saigo, K.; Morikawa, A.; Mukaiyama, T. Chem. Lett. 1975, 145. DOI:10.1246/cl.1975.145
・Saigo, K.; Morikawa, A.; Mukaiyama, T. Bull Chem. Soc. Jpn. 1976, 49, 1658. DOI:10.1246/bcsj.49.1656
・Ueno, Y.; Okawara, M. Tetrahedron Lett. 1976, 17, 4597. DOI:10.1016/S0040-4039(00)93941-8
・David, S.; Hanessian, S. Tetrahedron 1985, 41, 643. DOI:10.1016/S0040-4020(01)96443-9
・Grindley, T. B.; Thangarasa, R. Can. J . Chem. 1990, 68, 1007. DOI:10.1139/v90-158
・Martinelli, M. J. et al. Org. Lett. 1999, 1, 447. DOI: 10.1021/ol990658l
・Martinelli, M. J. et al. J. Am. Chem. Soc. 2002, 124, 3578. DOI: 10.1021/ja016031r
-
Reaction mechanism
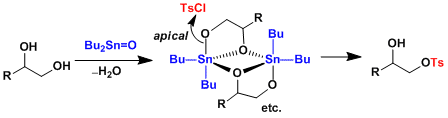
The dibutylstannylene with 1,2-diols formed its dimeric structure, Each of the tin atoms is in the center of a trigonal by pyramid with the butyl groups occupying two equatorial positions. One of the O-atoms (primary alcohol) is in the apical position and the other (secondary alcohol) in the equatorial position. The reactivity can be explained the steric effects between the apical position and equatorial position. The apical position is more sterically-unhindered than equatorial position, therefore the reaction proceeds at the apical position.
-
Example of reactions
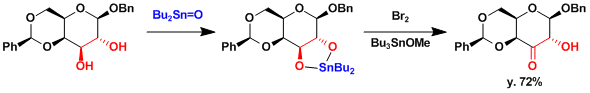
The selective oxidation of secondary alcohols has been achieved by the treatment with dibutyltin oxide, followed by bromine.
In the total synthesis of vinblastine, Fukuyama and coworkers utilized the mono tosylation of 1,2-diol (triol).[1] Upon treatment of the triol with TsCl and triethylamine in the presence of dibutyltin oxide, tosylation occurred selectively at the primary alcohol of the 1,2-diol in 84% yield.
-
Bibliography
[1] Yokoshima, S.; Ueda, S.; Kobayashi, S.; Sato, T.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T. J. Am. Chem. Soc. 2002, 124, 2137. DOI: 10.1021/ja0177049