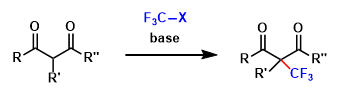
- Versatility
- Price
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
Efficient transfer of the trifluoromethyl group from a reagent to a target molecule is key for the reaction, and the reagents are classified according to their radical, nucleophilic or electrophilic character. In 1984 Yagupolskii and co-workers discovered that S-(trifluoromethyl)diarylsulfonium salts are effective for the electrophilic trifluoromethylation of thiophenolates.[a] Since this pioneering work, the design and synthesis of electrophilic trifluoromethylating reagents have been extensively investigated. Currently, the several electrophilic trifluoromethylating reagents are known such as Umemoto’s reagent, Togni’s reagent, Shibata-Johnson reagent, Langlois’ reagent and Baran’s reagent.
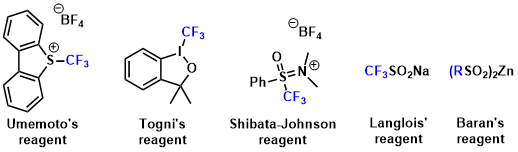
-
Literature reference
[a] Yagupolskii, L. M.; Kondratenko, N. V.; Timofeeva, G. N. J. Org. Chem. USSR 1984, 20, 103. [Link]
<General Reviews>
・Umemoto T. Chem. Rev. 1996,96, 1757. DOI: 10.1021/cr941149u
・Ma, J.-A.; Cahard, D. Chem. Rev. 2004, 104, 6119. DOI: 10.1021/cr030143e
・Ma, J.-A.; Cahard, D. J. Fluor. Chem. 2007, 128, 975. doi:10.1016/j.jfluchem.2007.04.026
・Shibata, N.; Matsnev, A.; Cahard, D. Beil. J. Org. Chem. 2010, 6, 65. doi:10.3762/bjoc.6.65
<Umemoto’s reagent>
・Umemoto T, Ishihara S. Tetrahedron Lett. 1990, 31, 3579. doi:10.1016/S0040-4039(00)94447-2
・Umemoto T, Ishihara S. J. Am. Chem. Soc. 1993, 115, 2156. DOI: 10.1021/ja00059a009
・Umemoto, T.; Adachi, K.; Ishihara, S. J. Org. Chem. 2007, 72, 6905. DOI: 10.1021/jo070896r
・Li, H. Synlett 2012, 2289. DOI: 10.1055/s-0032-1317176
<Togni’s reagent>
・Eisenberger, P.; Gischig, S.; Togni, A. Chem. Eur. J. 2006, 12, 2579. DOI: 10.1002/chem.200501052
・Kieltsch, I,; Eisenberger, P.; Togni, A. Angew. Chem. Int. Ed. 2007, 46, 754. DOI:10.1002/anie.200603497
・Eisenberger, P.; Kieltsch, I.; Armanino, N.; Togni, A. Chem. Commun. 2008, 1575. DOI:10.1039/B801424H
・Stanek, K.; Koller, R.; Togni, A. J. Org. Chem. 2008, 73, 7678. DOI: 10.1021/jo8014825
・Koller, R.; Stanek, K.; Stolz, D.; Aardoom, R.; Niedermann, K.; Togni, A. Angew. Chem. Int. Ed.2009, 48, 4332. DOI: 10.1002/anie.200900974
・Eisenberger, P.; Kiltsch, I.; Koller, R.; Stanek, K.; Togni, A. Org. Synth. 2011, 88, 168. [PDF]
<Shibata-Johnson reagent>
・Noritake, S.; Shibata, N.; Nakamura, S.; Toru, T.; Shiro, M. Eur. J. Org. Chem. 2008, 3465. DOI:10.1002/ejoc.200800419
<Langlois/Baran’s reagent>
・Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525. doi:10.1016/0040-4039(91)80524-A
・Ji, Y.; Bruecki, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S.Proc. Natl. Acad. Sci. USA 2011, 108, 14411. DOI: 10.1073/pnas.1109059108
・Fujiwara, Y.; Dixon, J.A.; Rodriguez, R.A.; Baxter, R.D.; Dixon, D.D.; Collins, M.R.; Blackmond, D.G.; Baran, P.S. J. Am. Chem. Soc. 2012, 134, 1494. DOI: 10.1021/ja211422g
・Fujiwara, Y.; Dixon, J.A.; O’Hara, F.; Daa Funder, E.; Dixon, D.D.; Rodriguez, R.A.; Baxter, R.D.; Herle, B.; Sach, N.; Collins, M.R.; Ishihara, Y.; Baran, P.S. Nature 2012, 492, 95. doi:10.1038/nature11680
・Ye, Y.; Kunzi, S. A.; Sanford, M. S. Org. Lett. 2012, 14, 4979. DOI: 10.1021/ol3022726
-
Related Books
[amazonjs asin=”0471187119″ locale=”US” title=”Molecular Structure and Energetics, Fluorine-Containing Molecules (Volume 8)”][amazonjs asin=”3527331662″ locale=”US” title=”Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications”][amazonjs asin=”352730617X” locale=”US” title=”Handbook of Fluorous Chemistry”]
-
Related Links
・Nucleophilic and Electrophilic Trifluoromethylation (PDF) ・フッ素化反応(Sigma-Aldrich, PDF) ・Trifluoromethylator(Sigma-Aldrich) ・Trifluoromethylation - Wikipedia ・Sodium trifluoromethanesulfinate - Wikipedia ・Fluorination Strategies and Methodologies (PDF)