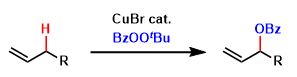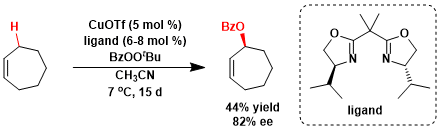- Popularity
- Reliability
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
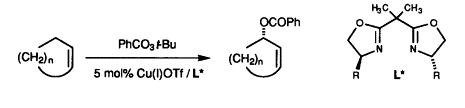
Kharasch and Sosnovsky reported the allylic oxidation of alkenes to give racemic allylic benzoate’s. This could be achieved efficiently using a tert-butyl perester as the oxidant, in the presence of a copper or cobalt salt.
They originally showed that allylic oxidation of alkenes, such as cyclohexene, could efficiently occur using a tert-butyl perester as the oxidant, in the presence of a copper or cobalt salt to give allylic benzoates in good yield. In most cases, the reaction was more efficient using a copper salt.
-
Literature reference
・Kharasch, M. S.; Sosnovsky, G. J. Am. Chem. Soc. 1958, 80, 756. DOI: 10.1021/ja01536a062
・Rawlinson, D. J.; Sosnovsky, G. Synthesis 1972, 1. DOI: 10.1055/s-1972-21818
<Mechanism>
・Kochi, J. K.; Mains, H. E. J. Org. Chem. 1965, 30, 1862. DOI: 10.1021/jo01017a036
・Beckwith, A. L.; Zavitsas, A. A. J. Am. Chem. Soc. 1986, 108, 8230. DOI: 10.1021/ja00286a020
<Review>
・Andrus, M. B.; Lashley, J. C. Tetrahedron 2002, 58, 845. DOI:10.1016/S0040-4020(01)01172-3
-
Reaction mechanism
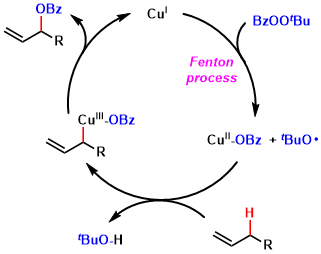
It was proposed that the reaction occurred via a radical mechanism, which was suggested to proceed in a concerted manner, with the copper center being intimately involved in the C–O bond forming step through the allyl cuprate. When the reaction was performed in the presence of an aliphatic acid, the ester of this acid was obtained rather than the corresponding benzoate.
-
Example of reactions
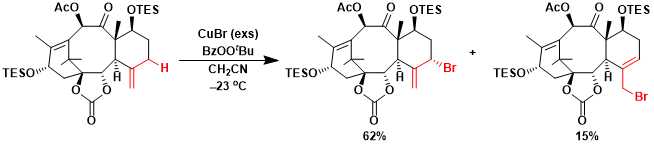
On the asymmetric synthesis of Taxol by Mukaiyama and coworkers, allylic bromination at the C-5 position of the starting material with excess amounts of CuBr and PhCO3tBu (1:1 molar ratio) gave the separable allylic bromides A and B in 62% and 15% yields, respectively.[1] Furthermore, on treating the allylic bromide A with CuBr in CH3CN at 50ºC, 25% of A and 69% of B were obtained, since A and B were in equilibrium under thermodynamic conditions.
In view of the potential utility of such a synthetic transformation, it does seem remarkable that the first synthetically useful asymmetric variant was only reported in the mid 1990s, independently by the groups of Pfaltz, Andrus, and Katsuki.
Pfaltz and coworkers reported enantioselective Kharasch-Sosnovsky oxidation by using chiral bisoxazoline-copper complexes as a catalyst.[2] Using 5 tool% of catalyst (copper(I) triflate/ chiral bisoxazoline), based on perester, 2-cyclohptenyl benzoate was formed in 44% yield with 82% ee. Depending on the substrate and the specific ligand, either acetonitrile or acetone proved to be the solvent of choice, the reaction in acetone being generally faster than in acetonitrile.
-
Procedure
Synthesis of taxol synthetic intermediate [1]
To a mixture of copper(i) bromide (113 mg, 0.709 mmol) and tert-butyl perbenzoate (192 mg, 0.790 mmol) at room temperature was added acetonitrile (6 mL). The reaction mixture was stirred for 45 min at 50 0ºC in darkness and then cooled down to room temperature. The copper reagent in acetonitrile thus prepared was immediately used in the following reaction.
The copper reagent in acetonitrile (6 mL) was added to a solution of ketone 60 (26.2 mg, 39.5 mmol) in acetonitrile (3 mL) at 23 0 ºC,. After the reaction mixture had been stirred for 12 h at 23 0ºC,, copper(i) bromide (157 mg, 1.185 mmol) was added. The reaction mixture was stirred for 1 h at 23 VC, and then the crude product was purified by column chromatography to afford allylic bromide 61 (18.2 mg, 62 %) as a pale yellow solid and allylic bromide 62 (4.4 mg, 15 %) as a pale yellow oil.
(S)-2-cyclohptenyl benzoate[2]
A solution of ligand (202mg, 0.75 mmol) in chloroform (2ml) was added to [CulOTf •0.5 (C6H6)] (140 mg, 0.5 mmol; Fluka pract., -90%) under N2. The solution was stirred at r.t. for 1 h. The catalyst solution was transferred through a Chromafd® disposable filter (pore diameter 0.2 µm) to a Schlenk tube containing cyclopentene (2.7 g, 40 mmol, 4 equiv, based on perester) and acetone (6 ml) under N2. ten-Butyl perbenzoate (2.03g, 9.4 mmol; Fluka pratt. -90%) was slowly added within 10 min and the reaction mixture was then kept at 0 °C for 14 days. After addition of water, the mixture was extracted twice with Et20. The organic layers were washed with 2N HCI and with water. Removal of the solvent in vacuo and purification by flash chromatography on silica gel (3.5 x 30 cm column, hexane/EtOAc 99:1) gave 1.10g of (S)-2-cyclopentenyl benzoate (56% yield, 76% based on consumed perester; 75% ee).
-
Bibliography
[1] Mukaiyama, T. et al. Chem. Eur. J. 1999, 5, 121. [abstract]
[2] “Enantioselective Allylic Oxidation Catalyzed by Chiral Bisoxazoline-Copper Complexes “
Gokhale, A. S.; Minidis, A. B. E.; Pfaltz, A. Tetrahedron Lett. 1995, 36, 1831. DOI:10.1016/0040-4039(95)00140-8
Copper(I) complexes prepared in situ from chiral bisoxazolines and Cu(I)OTf have been studied as catalysts for the allylic oxidation of cycloalkenes. Using 5 mol% of catalyst and tertbutyl perbenzoate as oxidant, optically active 2-cycloalkenyl benzoates were obtained in moderate to good yields. The highest enantiomeric excesses, 74% at 23 °C and up to 84% at lower temperatures, were observed for cyclopentene and cycloheptene, while cyclohexene gave somewhat lower selectivities ranging between 64–77%ee.
-
Related Books
[amazonjs asin=”3527323201″ locale=”US” title=”Modern Oxidation Methods”][amazonjs asin=”1119953278″ locale=”US” title=”Handbook of Reagents for Organic Synthesis: Catalytic Oxidation Reagents (Hdbk of Reagents for Organic Synthesis)”][amazonjs asin=”1466506016″ locale=”US” title=”Oxidation in Organic Synthesis”]
-
Related Links
・Kharasch reaction and related transformation (Baran's group, PDF) ・Kharasch-Sosnovsky Reaction (PDF) ・Kharasch-Sosnovsky Reaction - Wikipedia