Overall Score3.5
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
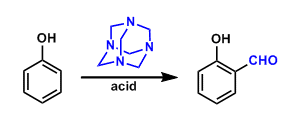
The formylation of phenols and anilines using hexamethylenetetramine is known as the Duff reaction.
-
General References
- Duff, J. C.; Bills, E. J. J. Chem. Soc. 1932, 1987; 1934, 1305; 1941, 547; 1945, 276.
- Ferguson, L. N. Chem. Rev. 1946, 38, 230. doi:10.1021/cr60120a002
- Ogata, Y.; Kawasaki, A.; Sugiura, F. Tetrahedron 1968, 24, 5001. doi:10.1016/S0040-4020(01)88408-8
- Wada, F. et al. Bull. Chem. Soc. Jpn. 1980, 53, 1473. doi:10.1246/bcsj.53.1473
- Smith, W. E. J. Org. Chem. 1972, 37, 3972. DOI: 10.1021/jo00797a057
- Larrow. J. F. et al. J. Org. Chem. 1994, 59, 1939. DOI: 10.1021/jo00086a062
- Lindoy, L. F. Synthesis 1998, 1029. DOI: 10.1055/s-1998-2110
-
Reaction Mechanism
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
- Gattermann-Koch Reaction
- Bouveault/Bodroux-Chichibabin Aldehyde Synthesis
- Reimer-Tiemann Reaction
- Vilsmeier-Haack Reaction
-
Related Books
-
External Links
- Duff reaction- Wikipedia