- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
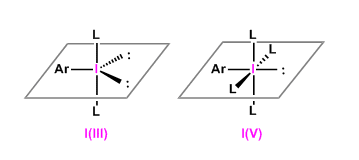
General Characteristics
Iodine can exist in high valence states because of its higher polarizability and smaller electronegativity compared with other halogen elements.
Hypervalent iodine compounds are readily reduced to stable monovalent state with the dissociation of apical groups, thus they act as good oxidizing agents.
Synthetically useful trivalent iodine reagents (λ3-iodane) include the following:
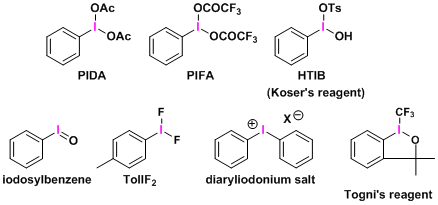
Pentavalent iodine (λ5-iodane) and higher valence states (such as iodine(VII)) are also known, among which the Dess-Martin reagent and periodate salt are commonly used as mild oxidizing agents.
-
General References
<Reviews>
- Valvoglis, A. Synthesis 1984, 709.
- Stang, P. F.; Zhdankin, V. V. Chem. Rev. 1996, 96, 1123. DOI: 10.1021/cr940424+
- Varvoglis, A.; Spyroudis, S. Synlett 1998, 221. DOI: 10.1055/s-1998-1619
- Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523. doi:10.1021/cr010003+
- Moriarty, R. M. J. Org. Chem. 2005, 70, 2893. doi:10.1021/jo050117b
- Wirth, T. Angew. Chem. Int. Ed. 2005, 44, 3656. DOI: 10.1002/anie.200500115
- Deprez, N. R.; Sanford, M. S. Inorg. Chem. 2007, 46, 1924. doi:10.1021/ic0620337
- Zhdankin, V. V. Chem. Rev. 2008, 108, 5299. doi:10.1021/cr800332c
- Ochiai, M.; Miyamoto, K. Eur. J. Org. Chem. 2008, 4229. DOI: 10.1002/ejoc.200800416
- Merritt, E. A.; Olofsson, B. Angew. Chem. Int. Ed. 2009, 48, 9052. DOI: 10.1002/anie.200904689
- Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073. DOI: 10.1039/b821747e
- Uyanik, M.; Ishihara, K. Chem. Commun. 2009, 2086. DOI: 10.1039/b823399c
-
Reaction Mechanism
The reactivity of hypervalent iodine compounds generally increases with the degree of how easily the apical groups dissociate.
-
Examples
Asymmetric oxidation using a chiral hypervalent iodine reagent.[1]
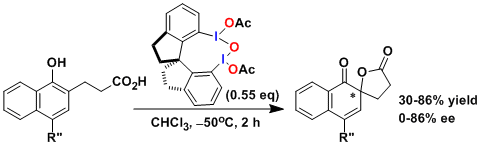
Progress has been made in recent years to reduce the amount of iodine reagents to substoichiometric.
-
Experimental Procedure
-
Experimental Tips
-
References
(1) Dohi, T.; Maruyama, A.; Takenaga, N.; Senami, K.; Minamitsuji, Y.; Fujioka, H.; Carmmerer, S. B.; Kita, Y. Angew. Chem. Int.Ed. 2008, 47, 3787. doi:10.1002/anie.200800464
-
Related Reactions
-
Related Books
[amazonjs asin=”3540441077″ locale=”US” title=”Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis (Topics in Current Chemistry)”]
[amazonjs asin=”0127149759″ locale=”US” title=”Hypervalent Iodine in Organic Synthesis (Best Synthetic Methods)”]
-
External Links
- Hypervalent Iodine Chemistry (organic-chemistry.org)
- Periodinane – Wikipedia
- The Chemistry of Hypervalent Iodine (PDF, from the MacMillan group)