Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
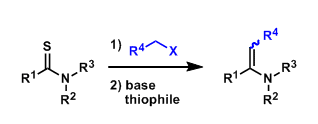
The Eschenmoser coupling begins with the S-alkylation of thioamides and gives enamine products (vinylogous amides, urethanes, etc.). As shown below, thiophiles (e.g. triphenylphosphine) are used to eliminate the sulfur.
-
General References
- Fischli, A.; Eschenmoser, A. Angew. Chem. Int. Ed. 1967, 6, 866. DOI: 10.1002/anie.196708661
- Roth, M.; Dubs, P.; Gotschi, E.; Eschenmoser, A. Helv. Chim. Acta 1971, 54, 710. DOI: 10.1002/hlca.19710540229
- Shiosaki, K. Comp. Org. Syn. 1991, 2, 865.
-
Reaction Mechanism

-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
- Barton-Kellogg Reaction
- Lawesson’s Reagent
-
Related Books
-
External Links