- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
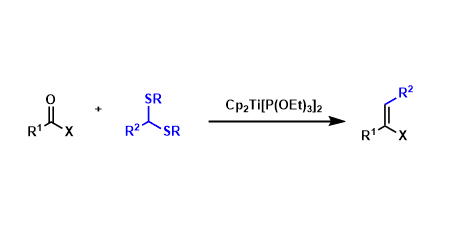
The olefination of thioacetals via generation of organotitanium intermediates using divalent titanocene reagents is known as the Takeda olefination.
The titanocene reagent needs to be prepared freshly for the reaction. Thioacetal-protected carbonyl compounds can be easily prepared. Compared with analogous systems such as the Tebbe, the Petasis, and the Takai-Lombardo reactions, the Takeda reaction has a wider substrate scope.
-
General References
Horikawa, Y.; Watanabe, M.; Fujiwara, T.; Takeda, T. J. Am. Chem. Soc. 1997, 119, 1127. DOI: 10.1021/ja962240d
Rahim, M. A.; Fujiwara, T.; Takeda, T. Tetrahedron 2000, 56, 763. doi:10.1016/S0040-4020(99)01091-1
Rahim, M. A.; Sasaki, H.; Saito, J.; Fujiwara, T.; Takeda, T. Chem. Commun. 2001, 625. DOI: 10.1039/B010156G
Hartley, R. C.; McKiernan, G. J. JCS Perkin Trans. 1 2002, 2763. doi: 10.1039/B009709H
-
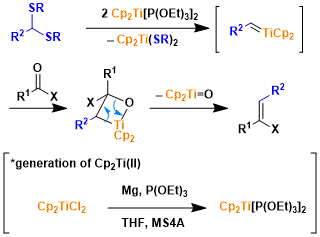
Reaction Mechanism
-
Examples
An example as a key step in ciquatoxin synthesis.[1]

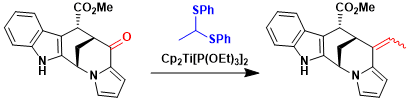
Total synthesis of alstoscholarine.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Uehara, H.; Oishi, T.; Inoue, M.; Shoji, M.; Nagumo, Y.; Kosaka, M.; Brazidec, J.-Y.; Hirama, M. Tetrahedron 2002, 58, 6493. doi:10.1016/S0040-4020(02)00660-9
[2] Gerfaud, T.; Xie, C.; Neuville, L.; Zhu, J. Angew. Chem. Int. Ed. 2011, 50, 3954. DOI: 10.1002/anie.201100257
-
Related Reactions
-
Related Books
[amazonjs asin=”352730634X” locale=”US” title=”Modern Carbonyl Olefination: Methods and Applications”]
-
External Links