Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
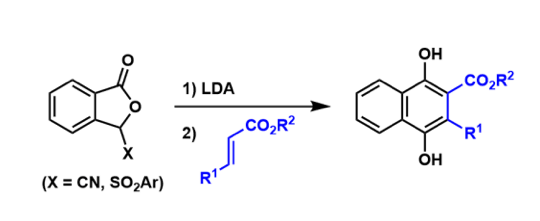
The synthesis of naphthalene hydroquinones from phthalide anions and α,β–undaturated carbonyl compounds through the Michael addition-then-Dieckmann condensation is known as the Hauser-Kraus annulation. It is used frequently to synthesize polyketide compounds.
Technically, the Hauser cyclization uses a sulfone group and the Kraus cyclization uses a cyano group at the benzylic position. The latter tends to be higher yielding because of the less steric hindrance.
When o-methyl benzoate esters are used instead of phthalides, the reaction is called the Staunton-Weinreb cyclization. Naphthols are the products in this case.
-
General References
- Hauser, F. M.; Rhee, R. J. Am. Chem. Soc. 1977, 99, 4533. DOI: 10.1021/ja00455a069
- Hauser, F. M.; Rhee, R. J. Org. Chem. 1978, 43, 178. DOI: 10.1021/jo00395a048
- Kraus, G. A.; Sugimoto, H. Tetrahedron Lett. 1978, 19, 2263. doi:10.1016/S0040-4039(01)91508-4
- Evans, G. E.; Leeper, F. J.; Murphy, J. A.; Staunton, J. J. Chem. Soc., Chem. Commun. 1979, 205. DOI: 10.1039/C39790000205
- Leeper, F. J.; Staunton, J. J. Chem. Soc. Chem. Commun. 1979, 206. DOI: 10.1039/C39790000206
- Dodd, J. H.; Weinreb, S. M. Tetrahedron Lett. 1979, 20, 3593. doi:10.1016/S0040-4039(01)95472-3
<reviews>
- Mitchell, A. S.; Russell, R. A. Tetrahedron 1995, 51, 5207. doi:10.1016/0040-4020(95)00247-6
- Mal, D.; Pahari, P. Chem. Rev. 2007, 107, 1892. DOI: 10.1021/cr068398q
- Rathwell, K.; Brimble, M. A. Synthesis 2007, 643. DOI: 10.1055/s-2007-965915
- Karmakar, R.; Pahari, P.; Mal, D. Chem. Rev. 2014, 114, 6213. DOI: 10.1021/cr400524q
- Donner, C. D. Tetrahedron 2013, 69, 3747. doi:10.1016/j.tet.2013.03.034
-
Reaction Mechanism

-
Examples
The reaction of homophthalide.[1]

Total synthesis of fused pentacyclic tetracyclin derivative.[2]

-
Experimental Procedure
-
Experimental Tips
-
References
- Tamura, Y.; Sasho, M.; Nakagawa, K.; Tsugoshi, T.; Kita, Y. J. Org. Chem. 1984, 49, 473. DOI: 10.1021/jo00177a017
- Charest, M. G.; Lerner, C. D.; Brubaker, J. D.; Siegel, D.; Myers, A. G. Science 2005, 308, 395. DOI:10.1126/science.1109755
-
Related Reactions
-
Related Books
-
External Links
- Robinson Annulation – Wikipedia
- Anionic Cyclization Reaction (Myers’ group, PDF)