- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
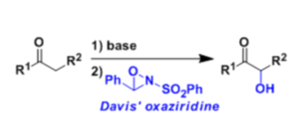
Ketones and esters can be hydroxylated at the α-position when their enolates are treated with 2-sulfonyloxaziridine (N-sulfonyloxaziridine, the Davis reagent).
Asymmetric α-hydroxylation is also possible using the chiral oxaziridine derived from camphor sulfonic acid.
-
History
In 1978, Professor Franklin Davis and his coworkers at Drexel University (currently at Temple University) reported the synthesis of 2-sulfonyloxaziridine, which is the most widely used oxaziridine reagent today.

-
General References
- Davis, F. A.; Jenkins, R., Jr.; Yocklovich, S. G. Tetrahedron Lett. 1978, 19, 5171. doi:10.1016/S0040-4039(01)85841-X
- Davis, F. A.; Vishwakarma, L. C.; Billmers, J. G.; Finn, J. J. Org. Chem. 1984, 49, 3241. DOI: 10.1021/jo00191a048
- Davis, F. A.; Sheppard, A. C.; Chen, B.-C.; Serajul Haque, M. J. Am. Chem. Soc. 1990, 112, 6679. DOI: 10.1021/ja00174a035
- Davis, F. A.; Chen, B. C. Tetrahedron Lett. 1990, 31, 6823. doi:10.1016/S0040-4039(00)97181-8
- Davis, F. A.; Kumar, A.; Chen, B. C. Tetrahedron Lett. 1991, 32, 867. doi:10.1016/S0040-4039(00)92107-5
<reviews>
- Davis, F. A.; Chen, B. C.Chem. Rev. 1992, 92, 919. DOI: 10.1021/cr00013a008
- Edupuganti, R.; Davis, F. Org. Biomol. Chem. 2012. 10, 5021. DOI: 10.1039/C2OB25345C
-
Reaction Mechanism
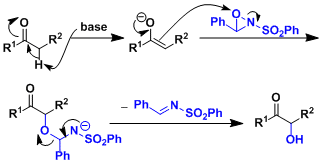
-
Examples
Example 1.[1]
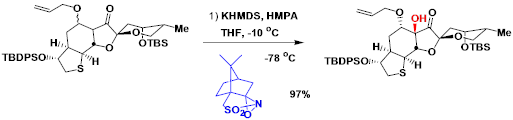
A diastereoselective example in the synthesis of (+)-jiadifenin.[2]
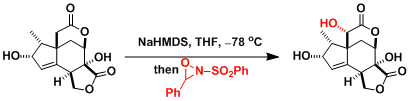
Another example in the synthesis of taxol.[3]

-
Experimental Procedure
Preparation of the Davis oxaziridine reagents.[4,5]
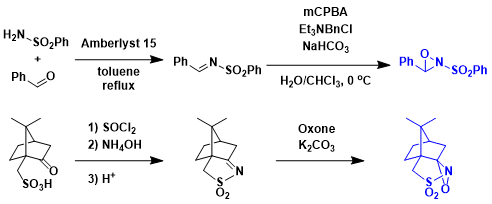
-
Experimental Tips
-
References
[1] Smith, A. B., III et al. J. Am. Chem. Soc. 1992, 114, 9419. DOI: 10.1021/ja00050a023
[2] Carcache, D. A.; Cho, Y. S.; Hua, Z.; Tian, Y.; Li, Y.-M.; Danishefsky, S. J. J. Am. Chem. Soc. 2006, 128, 1016. DOI: 10.1021/ja056980a
[3] Wender, P. A. et al. J. Am. Chem. Soc. 1997, 119, 2755 & 2757. DOI: 10.1021/ja9635387 DOI: 10.1021/ja963539z
[4] Vishwakarma, L. C.; Stringer, O. D.; Davis, F. A. Org. Synth. 1988, 66, 203. [PDF] [5] Towson , J. C.; Weismiller, M. C.; Lal, G. S.; Sheppard, A. C.; Kumar, A.; Davis, F. A. Org. Synth. 1990, 69, 158. [PDF]
-
Related Reactions
- Shi Asymmetric Epoxidation
- dimethyldioxirane
- Rubottom Oxidation
- Jacobsen-Katsuki Epoxidation
- Prilezhaev Epoxidation
-
Related Books
-
External Links
- The chemistry of oxaziridine (PDF)
- Oxidations (PDF)