Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
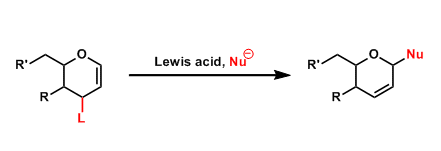
The introduction of nucleophilic groups to the 1-position of 1,2-glycals by an allylic substitution is called the Ferrier reaction. This reaction is referred to as the “Type I” Ferrier reaction, whereas the “Type II” Ferrier rearrangement is slightly different and known as the Petasis-Ferrier rearrangement.
-
General References
- Ferrier, R.J. J. Chem. Soc., Perkin Trans I 1979, 1455.
- Danishefsky, S. J.; Kerwin Jr., J. F. J. Org. Chem. 1982, 47, 3803. DOI: 10.1021/jo00140a053
- Danishefsky, S. J.; DeNinno, S.; Lartey, P. J. Am. Chem. Soc. 1987, 109, 2082. DOI: 10.1021/ja00241a028
- Ferrier, R. J.; Middleton, S. Chem. Rev. 1993, 93, 2779. DOI: 10.1021/cr00024a008
- Ferrier, R. J. Top. Curr. Chem. 2001, 215, 153.
- Ferrier, R. J.; Zubkov, O. A. Org. React. 2003, vol 62. doi:10.1002/0471264180.or062.04
-
Reaction Mechanism
The reaction is promoted by Lewis acids and proceeds through the formation of oxocarbenium species.

-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
-
Related Books
-
External Links
- Ferrier Rearrangement (Wikipedia)
- carbon-Ferrier Rearrangement