- Generality
- Reagent Availability
- Experimental User Friendliness
- Future Potential
- Criteria #5
-
General Characteristics
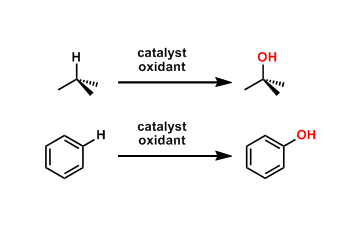
Catalytic C-H oxidation is a type of C-H activation reactions by which one can oxygenate unreactive C-H bonds, which are present ubiquitously in organic compounds.
The C-H bonds at allylic and benzylic positions and those adjacent to heteroatoms (e.g. O, N) are relatively susceptible to oxidation and there are many selective reactions for them.
On the other hand, direct oxygenation of less reactive aliphatic (sp3) and aromatic (sp2) C-H bonds remains one of the most challenging and competitive areas of research.
-
General References
- Ishihara, Y.; Baran, P. S. Synlett 2010, 1733. DOI: 10.1055/s-0030-1258123
- Roduner, E., Kaim, W., Sarkar, B., Urlacher, V. B., Pleiss, J., Gläser, R., Einicke, W.-D., Sprenger, G. A., Beifuß, U., Klemm, E., Liebner, C., Hieronymus, H., Hsu, S.-F., Plietker, B. and Laschat, S. ChemCatChem, 2013, 5, 82. DOI: 10.1002/cctc.201200266
-
Reaction Mechanism
-
Examples
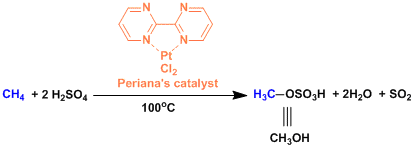
The Periana catalyst[1]: This catalyst is capable of oxidizing methane into methanol directly and selectively. Complete oxidation to acetic acid is also possible by selecting appropriate conditions.
There has been a substantial progress in the development of directed C-H oxidation and many of the examples are reasonably regioselective. The system developed by Yu is shown below.[2]
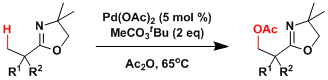
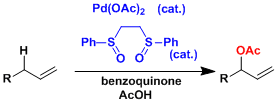
The White catalyst[3]: This catalyst selectively oxidizes allylic positions. The reaction conditions are remarkably mild and it can be used for the synthesis of complex molecules.
An application to the macrolactonization.[4]
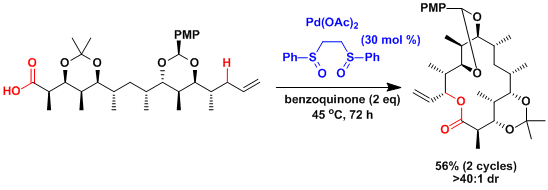
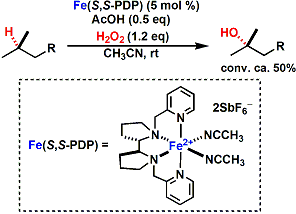
The White laboratory also reported the iron-catalyzed aliphatic C-H oxidation without directing groups.[5]
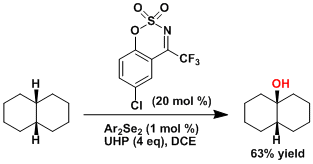
The hydroxylation of aliphatic C-H bonds using the electron-poor oxaziridine catalyst.[6]
Methyltrioxorhenium is known to act as an effective catalyst of C-H hydroxylation.[7]
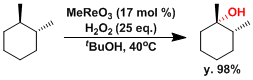
Ruthenium-catalyzed C-H oxidation.[8]
Chromic acid-catalyzed C-H oxidation at low temperatures.[9]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] (a) Perianam R. A. et al. Science 1998, 280, 560. DOI: 10.1126/science.280.5363.560 (b) Periana, R. A. et al. Science 2003, 301, 814. DOI: 10.1126/science.1086466 [2] Yu, J.-Q. et al. Angew. Chem. Int. Ed. 2005, 44, 7420. doi:10.1002/anie.200502767 [3] (a) Chen, M. S.; White, M. C. J. Am. Chem. Soc. 2004, 126, 1346. DOI: 10.1021/ja039107n (b) Chen, M. S.; Prabagaran, N.; Labenz, N. A.; White, M. C. J. Am. Chem. Soc. 2005, 127, 6970. DOI:
10.1021/ja0500198 [4] (a) Stang, E. M.; White, M. C. Nature Chem. 2009, 1, 547 DOI: 10.1038/NCHEM.351 (b) Fraunhoffer, K. F.; Prabagaran, N.; Sirois, L. E.; White, M. C. J. Am. Chem. Soc. 2006, 128, 9032. DOI: 10.1021/ja063096r [5] (a) Chen, M. S.; White, M. C. Science 2007, 318, 783. DOI: 10.1126/science.1148597 (b) Chen, M. S.; White, M. C. Science 2010, 327, 566 DOI: 10.1126/science.1183602
(c) Vermeulen, N. A.; Chen, M. S.; White, M. C. Tetrahedron 2009, 65, 3078. DOI: 10.1016/j.tet.2008.11.082 (d) Bigi, M. A.; Reed, S. A.; White, M. C., Nat. Chem. 2011, 3, 216. DOI: 10.1038/nchem.967 [6] du Bois, J. et al. J. Am. Chem. Soc. 2005, 127, 15391. DOI: 10.1021/ja055549i [7] (a) Wearing, J. T. et al. Tetrahedron Lett. 1995, 36, 6415. doi:10.1016/0040-4039(95)01344-H
(b) Hermann,W. A. et al. Angew. Chem. Int. Ed. Engl. 1993, 32, 1157. doi:10.1002/anie.199311571
[8] McNeill, E.; Bois, J. D., J. Am. Chem. Soc. 2010, 132, 10202. DOI: 10.1021/ja1046999 [9] (a) Lee, S.; Fuchs, P. L., J. Am. Chem. Soc. 2010, 124, 13978.(b) Lee, S.; Fuchs, P. L., Org. Lett. 2004, 6 ,1437. DOI: 10.1021/ol049712a
-
Related Reactions
- C-H Oxidation with NHPI Catalyst
- Kharasch-Sosnovsky Oxidation
- Fujiwara-Moritani Reaction
- Cross Dehydrogenative Coupling (CDC)
- Hofmann-Löffler-Freytag Reaction
- Catalytic C-H activation
- Du Bois Amination
- C-H Insertion of Metal Carbenoid
- Miyaura-Ishiyama-Hartwig Borylation
- Barton Reaction
- Selenium Dioxide
-
Related Books
[amazonjs asin=”3642094937″ locale=”US” title=”Directed Metallation (Topics in Organometallic Chemistry)”]
[amazonjs asin=”9048136970″ locale=”US” title=”Alkane C-H Activation by Single-Site Metal Catalysis (Catalysis by Metal Complexes)”]
[amazonjs asin=”3642084362″ locale=”US” title=”Activation of Unreactive Bonds and Organic Synthesis (Topics in Organometallic Chemistry)”]
-
External Links
- Alkane Hydroxylation (PDF, Phil Baran’s group)
- White Research Group