- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-The acylation of aromatic rings using acyl halides or acid anhydrides mediated by Lewis acids is called the Friedel-Crafts acylation. Arenes substituted with electron donating groups are more reactive than those substituted with electron withdrawing groups.
-While the Friedel-Crafts alkylation tends to suffer from problems such as overalkylation and rearrangement except for certain intramolecular cases, the F-C acylation is easier to be used intermolecularly since the electron-withdrawing property of the first acyl group slows down the second and further acylations.
-
General References
・Friedel, C.; Crafts, J. M. Compt. Rend. 1877, 84, 1450.
・Crafts, J. M.; Ador, E. Ber. 1877, 10, 2173.
・Crafts, J. M.; Ador, E. Bull. Soc. Chim. France 1880, 531.
・Price, C.C. Org. React. 1946, 3, 1.
・Gore, P. Chem. Rev. 1955, 55, 229. DOI: 10.1021/cr50002a001
・Groves, J. K. Chem. Soc. Rev. 1972, 1, 73. DOI: 10.1039/CS9720100073
・Eyley, S. C. Comprehensive Organic Synthesis 1991, 2, 707.
・Heaney, H. Comprehensive Organic Synthesis 1991, 2, 733.
-
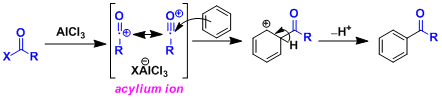
Reaction Mechanism
The reaction is an example of electrophilic aromatic substitution (SEAr). The highly electrophilic acylium ion formed from an acid halide and AlCl3 is considered to be the reactive species.
-
Examples
The Friedel-Crafts alkylation is inherently associated with the problem of overalkylation. To circumvent it, the F-C acylation is occasionally used in combination with subsequent deoxygenation (e.g. Clemmensen reduction).[1]
The synthesis of phenolphthalein.
-
Experimental Procedure
-
Experimental Tips
To quench the reaction, add the reaction mixture slowly into ice/water. The reverse (the addition of water to the reaction) would be dangerously exothermic.
-
References
[1] Lee, C. C.; Zohdi, H. F.; Sallam, M. M. M. J. Org. Chem. 1985, 50, 705. DOI: 10.1021/jo00205a034
[2] Patil, M. L. et al. Tetrahedron 2002, 58, 6615.
-
Related Books
[amazonjs asin=”3527310746″ locale=”US” title=”Handbook of C-H Transformations”]

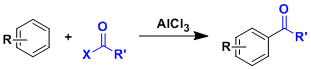
![FC_acyl_3[1]](https://assets.en.chem-station.com/uploads/2014/04/FC_acyl_31.gif)
![FC_acyl_4[1]](https://assets.en.chem-station.com/uploads/2014/04/FC_acyl_41.gif)
![FC_acyl_5[1]](https://assets.en.chem-station.com/uploads/2014/04/FC_acyl_51.gif)