- Generality
- Reagent Availabiltiy
- Safety and Environmental Impact
- Criteria #4
- Criteria #5
Abstract
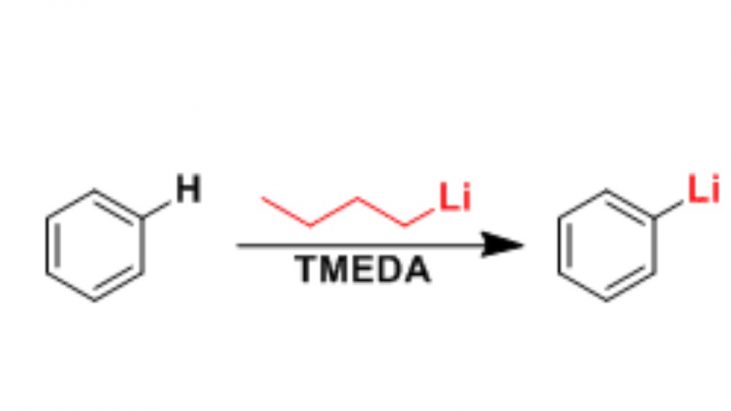
The reactivity of organometallic reagents is roughly determined by the degree of polarization of carbon-metal bonds, with carbon-lithium bonds exhibiting the greatest polarization (ionic bonding). For this reason, organolithium compounds are the most reactive organometallic reagents and are more reactive than typical organometallic reagents such as Grignard reagents and organozinc reagents. They are unstable in air and water. Organolithium reagents react with dioxygen and water violently with intense heat generation. Therefore, the reaction must be carried out under inert gas atmosphere, using dehydrated solvent, and at low temperature. It is commonly used as a metal exchange reagent, nucleophile, or strong base.
Basic Literature
- Chalk, A. J.; Hoogeboom, T. J. J. Organomet. Chem. 1968,11, 615. doi:10.1016/0022-328X(68)80091-9
- Review: Mallan, J. M.; Bebb, R. L. Chem. Rev. 1969, 69, 693. DOI: 10.1021/cr60261a006
- Review: Wu, G.; Huang, M. Chem. Rev. 2006, 106, 2596. DOI: 10.1021/cr040694k
- Review: Seyferth, D. Organometallics, 2006,25, 2. DOI: 10.1021/om058054a
Mechanism
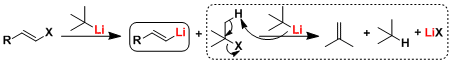
The lithium-halogen exchange reaction proceeds by single electron transfer (SET) mechanism or nucleophilic addition to halogen-elimination mechanism. (Reference: J. Organomet. Chem. 1988, 352, 1.)
Halogen-lithium exchange reaction using t-BuLi requires two equivalents of t-BuLi since t-BuLi is consumed in the reaction with the t-butyl halide produced after lithiation.
Textbook Knowledge
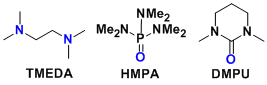
In solution, organolithiums exist in an aggregated state (oligomers). N,N,N’,N’-tetramethylethylenediamine (TMEDA), hexamethylphosphoramide (HMPA) and dimethylpropyleneurea (DMPU) are examples frequently used for dissociating aggregates.
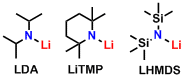
Organolithiums are both nucleophilic and basic. Side reactions due to its high reactivity for both properties are problematic. For the use of bases rather than nucleophiles, lithium amide reagents (prepared from bulky secondary amines and organolithiums) are used. Typical reagents include LDA (Lithium diisopropylamide), LiTMP (Lithium 2,2,6,6-tetramethylpiperidide), and LHMDS (Lithium hexamethyldisilazide).
On the other hand, for nucleophilic applications, nucleophilic additions and substitutions can be made with less basicity when used in the form of a copper art complex (R2CuLi). Lithium reagents are hard, and 1,2-addition takes precedence over 1,4-addition when using α,β-unsaturated carbonyl compounds as substrates. In contrast, 1,4-selective additions are possible with the use of copper ate complexes (cupurates).
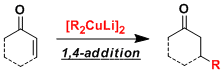
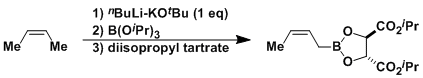
The combination of nBuLi-KOtBu is called the Schlosser-Lochmann base and works as a super strong base. It can be used, for example, for the deprotonation of hydrocarbons to the allylic position.[1] It is often used in the synthesis of optically active allylboranes.[2] Reported by Roush et al.:
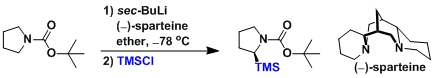
Enantioselective deprotonation can be achieved by adding (-)-Sparteine as a coordinating additive. An Example:[3]
Notes and Tips
Commonly used and commercially available representative reagents include MeLi, PhLi, n-BuLi, sec-BuLi, and t-BuLi.
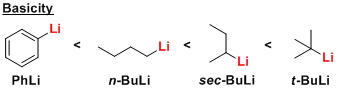
Commercially available lithium reagents are stored in Sure-sealed reagent bottles, but after two or three punctures with a syringe needle, a hole opens, and the seal becomes much less tight. Store them with several layers of Teflon seals or Parafilm. For more rigorous and longer-term storage, transfer the reagent to a Schlenk tube for storage.
Titrate the concentration at appropriate times. The diphenylacetic acid method [4] and the 2,2′-bipyridyl method [5] are known as simple and reliable methods.
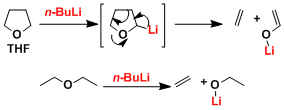
Although generally stable in hydrocarbon solvents such as hexane, organolithiums are known to gradually decompose in ether solvents via a β-elimination reaction (for example, the half-life of n-BuLi in THF at 0°C is 24 hours). The order of decomposability is DME > THF > diethyl ether.
Organolithiums, particularly t-BuLi, are highly reactive and frequently cause ignition accidents. Use with caution and always have fire extinguishing equipment on hand in case of large-scale reactions. Guide by experienced users is requested.[6]
References
[1] Schlosser, M. Pure Appl. Chem. 1988, 11, 1627.
[2] Roush, W. R.; Ando, K.; Powers, D. B.; Halterman, R. L.; Palkowitz, A. D. Tetrahedron Lett. 1988, 29, 5579. doi:10.1016/S0040-4039(00)80816-3
[3] Kerrik, S. T.; Beak, P. J. Am. Chem. Soc. 1991, 113, 9708. DOI: 10.1021/ja00025a066
[4] Kofron, W. G.; Baclawski, L. M. J. Org. Chem. 1976, 41, 1879. DOI: 10.1021/jo00872a047
[5] Watson, S. C.; Eastham, J. F. J. Organomet. Chem. 1967, 9, 165. doi:10.1016/S0022-328X(00)92418-5
[6] C&E news: https://cen.acs.org/safety/lab-safety/10-years-Sheri-Sangjis-death/97/i1
Related Reactions
Related Book
[amazonjs asin=”0080432611″ locale=”JP” title=”Organolithiums: Selectivity for Synthesis, Volume 23 (Tetrahedron Organic Chemistry)”][amazonjs asin=”1483233804″ locale=”JP” title=”The Chemistry of Organolithium Compounds”]