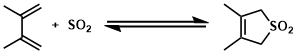- Popularity
- Versatility
- Gentleness
- Criteria #4
- Criteria #5
-
Characteristics
Cheletropic reactions are types of pericyclic reaction. A type of addition reaction in which a conjugated molecule forms two single bonds from terminal atoms of the conjugated system to a single atom on another molecule to give a cyclic adduct. One of the most synthetically important cheletropic reactions is the addition of a singlet carbene to an alkene to make a cyclopropane. The other examples: cycloaddition of SO2 to a diene, decarbonylation of cyclopropanone.
-
Literature reference
・Woodward, R.B.; Hoffman, R. Angew. Chem. Int. Ed. Engl. 1969, 8, 781. DOI:10.1002/anie.196907811
-
Example of reactions
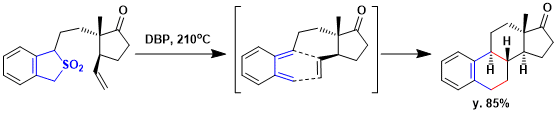
Alkylation of sulfone gives the requisite sulfone precursors and pyrolysis of the substituted sulfones to produce directly the polycyclic frameworks via o-quinodimethanes. e.g.) Thermolysis of the substituted sulfone at 210 ºC in di-n-butyl phthalate solution (ca. 0.5 M) generated the corresponding o-quinodimethanes by cheletropic elimination of SO2 and led to the indicated polycycles by intramolecular [4+2]cycloaddition.[1]
The Ramberg–Bäcklund reaction is an organic reaction converting an α-halo sulfone into an alkene in presence of a base with extrusion of sulfur dioxide. In the mechanism of extrusion of sulfur dioxide is one of the cheletropic reaction as well.
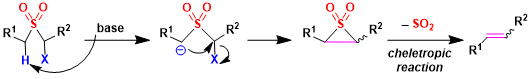
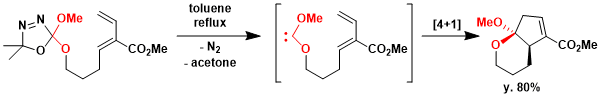
The [4 + 1]-cycloaddition between a carbene and a diene to give a cyclopentene derivative is, in a sense, the five-membered ring equivalent of the Diels-Alder reaction. Heating bis-heteroatom carbene, an oxadiazoline gave diastereomerically pure acetal in 80% yield.[2]
-
Procedure[2a]
Diene (80.0 mg, 0.27 mmol) was dissolved in toluene (5 mL). The reaction mixture was heated to reflux and stirred for 12h. The solvent was removed under vacuum to give a yellow oil. The crude product was purified by flash chromatography on a silica gel column eluting with ethyl acetate / hexanes (10%) to give 54 mg of a product as colorless oils in 80% yield.
-
Bibliography
[1] Nicolaou, K. C.; Barnette, W. E.; Ma, P. J. Org. Chem. 1980, 45, 1463. DOI: 10.1021/jo01296a023
[2] (a) Spino, C.; Rezaei, H.; Dupont-Gaudet, K.; Belanger, F. J. Am. Chem. Soc. 2004, 126, 9926. DOI: 10.1021/ja046344x (b) Boisvert , L.; Beaumier, F.; Spino, C. Org. Lett. 2007, 9, 5361. DOI:10.1021/ol702172t
-
Related Books
[amazonjs asin=”3527314393″ locale=”US” title=”Pericyclic Reactions – A Textbook: Reactions, Applications and Theory”][amazonjs asin=”8180941787″ locale=”US” title=”Organic Photochemistry and Pericyclic Reactions”]
-
Related Links
Cheletropic reaction - Wikipedia
Cheletropic reactions (Baran's group, PDF)