- Popularity
- Criteria #2
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
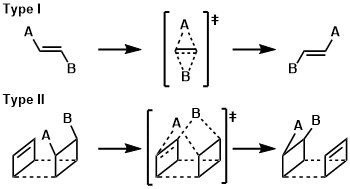
In 1972, M. T. Reetz originally defined dyotropic (from the greek dyo , meaning two) rearrangements as a new class of pericyclic valence isomerizations in which two σ -bonds simultaneously migrate intramolecularly.
A dyotropic reaction can be defined as an uncatalyzed process in which two o-bonds simultaneously migrate intramolecularly. Reactions in which the two o-bonds interchange their positions are of Type I. In Type I processes no direct positional interchange occurs . But, a variety of examples have since been reported in which the simultaneity of the two σ – bond migrations varies from synchronous (and concerted) to asynchronous (and concerted) to stepwise (separated by a discrete intermediate). Nowadays, dyotropic transformations are standard tools for the construction of organic and organometallic molecules.
-
Literature reference
・Reetz, M. T. Angew. Chem. Int. Ed. Engl. 1971, 11, 129. DOI:10.1002/anie.197201291
・Reetz, M. T. Angew. Chem. Int. Ed. Engl. 1971, 11, 130. DOI:10.1002/anie.197201311
・Reetz, M. Tetrahedron 1973, 29, 2189. DOI:10.1016/0040-4020(73)80163-2
・Fernandez, I.; Cossio, F. P.; Sierra, M. A. Chem. Rev. 2009, 109, 6687. DOI: 10.1021/cr900209c
・Gutierrez, O.; Tantillo, D. J. J. Org. Chem. 2012, 77 , 8845. DOI: 10.1021/jo301864h
-
Example of reactions
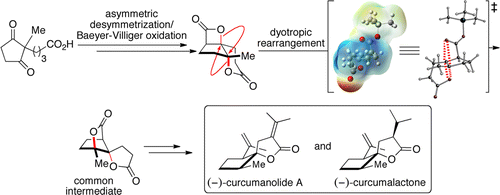
A Dyotropic rearrangement of a fused, tricyclic β – lactone proceeds via unprecedented stereospecific, 1,2-acyl migrations delivering bridged, spiro-γ -butyrolactones. In the presence of substoichiometric TMSOTf (15 mol %), dyotropic rearrangement slowly produced the desired spiro-γ -lactone in good yield (73%) with complete stereochemical fidelity (98% ee), The spiro-γ -lactone enabled rapid access to the core structures of curcumanolide A and curcumalactone.[1]
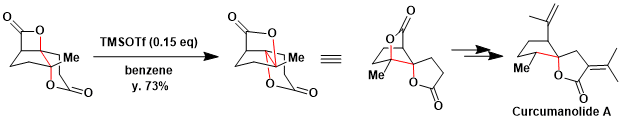
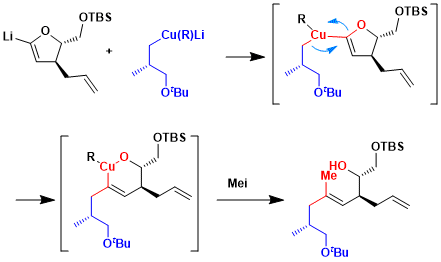
The synthesis of the C16– C23 fragment 311 of the potent immunosuppressive agent FK-506 was achieved by reaction of the lithiated dihydrofuran with the homocuprate.[2] This reaction forms the oxycuprate intermediate via the expected 1,2-dyotropic rearrangement, which affords the desired trisubstituted alkene in ca. 50% overall yield on quenching with MeI in the presence of HMPA.
These transformations exemplify the value of the method for the construction of trisubstituted alkenes in sterically encumbered environments.
-
Procedure[2]
To a magnetically stirred solution of (2R)-2-methylS-Fbutoxy-1-bdopropane (396 mg, 1.55 mmol) in EtsO (3 ml) at -76°C was added t-BuLi in pentane (1.7 M, 1.55 ml, 2.64 mmol). The mixture was warmed to 0°C and stirred for 10 min whereupon THF (0.5 ml) was added dropwise to destroy excess tBuLi. After 20 min at 0°C the mixture was cooled to 76°C and a solution of CuBrSMes (160 mg. 0.775 mmol) in SMe, (2 ml) was added dropwise. The mixture was allowed to slowly warm to room temperature during which time the mixture became a homogeneous yellow solution (at ca. -20°C).
The mixture was then heated to reflux whilst a solution of the lithiated dihydrofuran. After addition was complete the mixture was heated under reflux for 30 min and then cooled to -76 °C whereupon HMPA (95 µl, 0.775 mmol) was added followed by Mel (177 mg, 1.16 mmol). The mixture was then stirred at -40°C for 1 h and allowed to attain mom temperature over 1 h. The reaction mixture was poured into saturated aqueous NH&I and the product extracted into Et20. The extract was dried over MgSO4, concentrated in vacua, and the residue purified by colymn chromatography on silica gel 60 eluting with 5% Et20 in hexane to give product (69 mg, 57%) as a colorless oil.
-
Bibliography
[1]”Dyotropic Rearrangements of Fused Tricyclic β-Lactones: Application to the Synthesis of (−)-Curcumanolide A and (−)-Curcumalactone”
Leverett, C. A.; Purohit, V. C.; Johnson, A. G.; Davis, R. L.; Tantillo, D. J. Romo, D. J. Am. Chem. Soc. 2012, 134, 13348. DOI: 10.1021/ja303414a
Dyotropic rearrangements of fused, tricyclic β-lactones are described that proceed via unprecedented stereospecific, 1,2-acyl migrations delivering bridged, spiro-γ-butyrolactones. A unique example of this dyotropic process involves a fused bis-lactone possessing both β- and δ-lactone moieties which enabled rapid access to the core structures of curcumanolide A and curcumalactone. Our current mechanistic understanding of the latter dyotropic process, based on computational studies, is also described. Other key transformations in the described divergent syntheses of (−)-curcumanolide A and (−)-curcumalactone from a common intermediate (11 and 12 steps from 2-methyl-1,3-cyclopentanedione, respectively), include a catalytic, asymmetric nucleophile (Lewis base)-catalyzed aldol-lactonization (NCAL) leading to a tricyclic β-lactone, a Baeyer–Villiger oxidation in the presence of a β-lactone, and highly facial-selective and stereocomplementary reductions of an intermediate spirocyclic enoate. The described dyotropic rearrangements significantly alter the topology of the starting tricyclic β-lactone, providing access to complex spirocyclic cyclopentyl-γ-lactones and bis-γ-lactones in a single synthetic operation.
[2] “A synthesis of the C16-C23 segment of FK-506”
Stocks, M.; Kocienski, P.; Donald, D. K. Tetrahedron Lett. 1990, 31, 1637. DOI: 10.1016/0040-4039(90)80037-M
A copper-catalysed migratory insertion reaction was used to construct the tri-substituted alkene of the C16-C23 segment of the potent immunosuppressant FK-506 (Tsukubaenolide).
-
Related Books
[amazonjs asin=”3527332944″ locale=”US” title=”Tautomerism: Methods and Theories”][amazonjs asin=”3527314393″ locale=”US” title=”Pericyclic Reactions – A Textbook: Reactions, Applications and Theory”]
-
Related Links
Dyotropic reaction - Wikipedia Dyotropic rearrangement (Denmark's Group, PDF)