- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Sulfur dioxide (SO2) is a synthetically useful reagent as a source of sulfur but it has to be used with care in a well-ventilated hood since it is a toxic gas.
DABSO, the charge-transfer complex composed of SO2 and DABCO, can be used more safely as an equivalent of SO2. It is hygroscopic but can be handled easily and is commercially available.
By treating DABSO with Grignard reagents or under palladium-catalyzed coupling conditions, various sulfonic acid derivatives can be synthesized.
-
General References
- Santos, P. S.; Mello, M. T. S. J. Mol. Struct. 1988, 178, 121. doi:10.1016/0022-2860(88)85010-5
- Nguyen, B.; Emmett, E. J.; Willis, M. C. J. Am. Chem. Soc. 2010, 132, 16372. doi:10.1021/ja1081124
- Woolven, H.; Gonzalez-Rodriguez, C.; Marco, I.; Thompsono, A. L.; Willis, M. C. Org. Lett. 2011, 13, 4876. DOI: 10.1021/ol201957n
<REVIEWS>
- Martial, L. Synlett 2013, 24, 1595. DOI: 10.1055/s-0033-1339301
- Emmett, E. J.; Willis, M. C. Org. Synth. 2014, 91, 125. DOI: 10.15227/orgsyn.091.0125
- Bisseret, P.; Bianchard, N. Org. Biomol. Chem. 2013, 11, 5393. DOI: 10.1039/C3OB40997J
- Emmett, E. J.; Willis, M. C. Asian J. Org. Chem. 2015, 4, 602. DOI: 10.1002/ajoc.201500103
-
Reaction Mechanism
-
Examples
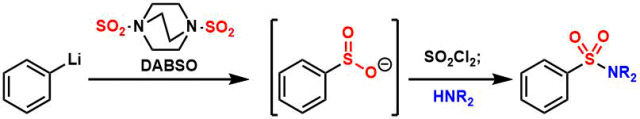
The examples of sulfonamide and sulfone synthesis.[1]

One-step synthesis of sulfonylhydrazine under palladium-catalyzed conditions.[2]

One-step synthesis of sulfonamide[3]: The yield tends to be low for substrates derived from non-aromatic ketones. K2S2O5 can be used in place of DABSO.

-
Experimental Procedure
Preparation of DABSO: The commercial price of DABSO is high, but a procedure to synthesize it from the Karl-Fischer reagent without SO2 gas has been reported.[4]
 An oven-dried 500-mL round-bottomed flask containing a 5 cm Teflon-coated oval stir bar is fitted with a rubber septum and allowed to cool to room temperature under vacuum. At room temperature (22 °C), 1,4-diazabicyclo[2,2,2]octane (DABCO) (15.0 g, 134 mmol, 1 equiv) is added and the flask is connected to a vacuum line via a needle inserted through the septum. The flask is evacuated then back-filled with argon. This process is repeated three times. Dry THF (180 mL) is introduced via syringe under a positive argon pressure. After complete dissolution, the reaction medium is ice-cooled and Karl-Fischer reagent (solution A, 120 mL, ~280 – 375 mmol SO2) is added dropwise via syringe at 0 °C (external temperature) over 30 min. Precipitation of DABSO is observed shortly after the addition is initiated. After stirring 30 min at 0 °C, the ice-bath is removed and the suspension is stirred at room temperature for an additional 3 h. The suspension is filtered through a 250-mL sintered-glass funnel under reduced pressure (vacuum pump, 15 mmHg). The white solid is washed on the funnel by triturating a few seconds with diethyl ether (50 mL) without suction. The collected solid is transferred back to the original 500-mL flask. Diethyl ether (200 mL) is added and the suspension is stirred at room temperature for 15 min under a nitrogen atmosphere. The solid is collected on the same sintered-glass funnel. The re-slurry procedure is repeated twice in the same manner. Since DABSO is a hygroscopic solid, it is immediately transferred into a 250-mL round-bottomed flask and dried in a desiccator under vacuum for 12 h to afford DABSO (30.9-31.0 g, 96-97%) as a colorless powder.
An oven-dried 500-mL round-bottomed flask containing a 5 cm Teflon-coated oval stir bar is fitted with a rubber septum and allowed to cool to room temperature under vacuum. At room temperature (22 °C), 1,4-diazabicyclo[2,2,2]octane (DABCO) (15.0 g, 134 mmol, 1 equiv) is added and the flask is connected to a vacuum line via a needle inserted through the septum. The flask is evacuated then back-filled with argon. This process is repeated three times. Dry THF (180 mL) is introduced via syringe under a positive argon pressure. After complete dissolution, the reaction medium is ice-cooled and Karl-Fischer reagent (solution A, 120 mL, ~280 – 375 mmol SO2) is added dropwise via syringe at 0 °C (external temperature) over 30 min. Precipitation of DABSO is observed shortly after the addition is initiated. After stirring 30 min at 0 °C, the ice-bath is removed and the suspension is stirred at room temperature for an additional 3 h. The suspension is filtered through a 250-mL sintered-glass funnel under reduced pressure (vacuum pump, 15 mmHg). The white solid is washed on the funnel by triturating a few seconds with diethyl ether (50 mL) without suction. The collected solid is transferred back to the original 500-mL flask. Diethyl ether (200 mL) is added and the suspension is stirred at room temperature for 15 min under a nitrogen atmosphere. The solid is collected on the same sintered-glass funnel. The re-slurry procedure is repeated twice in the same manner. Since DABSO is a hygroscopic solid, it is immediately transferred into a 250-mL round-bottomed flask and dried in a desiccator under vacuum for 12 h to afford DABSO (30.9-31.0 g, 96-97%) as a colorless powder.
-
Experimental Tips
-
References
- Woolven, H.; Gonzalez-Rodriguez, C.; Marco, I.; Thompsono, A. L.; Willis, M. C. Org. Lett. 2011, 13, 4876. DOI: 10.1021/ol201957n
- Nguyen, B.; Emmett, E. J.; Willis, M. C. J. Am. Chem. Soc. 2010, 132, 16372. doi:10.1021/ja1081124
- Tsai, A. S. et al. Org. Lett. 2016, 18, 508. doi:10.1021/acs.orglett.5b03545
- Martial, L; Bischoff, L. Org. Synth. 2013, 90, 301. DOI: 10.15227/orgsyn.090.0301
-
Related Reactions
-
Related Books
[amazonjs asin=”B00W89DXK0″ locale=”US” title=”The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine”]
[amazonjs asin=”3527330151″ locale=”US” title=”Bioisosteres in Medicinal Chemistry, Volume 54″]
-
External Links