- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
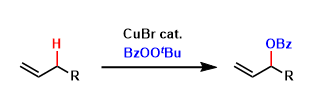
Allylic C-H oxidation can be effected using acylperoxide under copper-catalyzed conditions. The alkene starting materials are usually used in excess. The terminal olefins are formed as major products.
-
General References
- Kharasch, M. S.; Sosnovsky, G. J. Am. Chem. Soc. 1958, 80, 756. DOI: 10.1021/ja01536a062
- Rawlinson, D. J.; Sosnovsky, G. Synthesis 1972, 1. DOI: 10.1055/s-1972-21818
<Mechanism>
- Kochi, J. K.; Mains, H. E. J. Org. Chem. 1965, 30, 1862. DOI: 10.1021/jo01017a036
- Beckwith, A. L.; Zavitsas, A. A. J. Am. Chem. Soc. 1986, 108, 8230. DOI: 10.1021/ja00286a020
< Review> - Andrus, M. B.; Lashley, J. C. Tetrahedron 2002, 58, 845. doi:10.1016/S0040-4020(01)01172-3
-
Reaction Mechanism
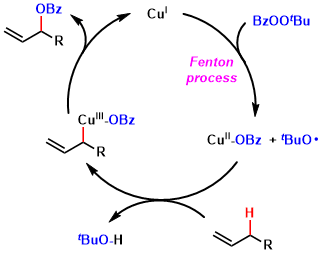
The proposed mechanisms starts with the formation of the oxyl radical species from acylperoxide and copper(I) (process known as the Fenton reaction), which then cleaves the allylic C-H bond.
-
Examples
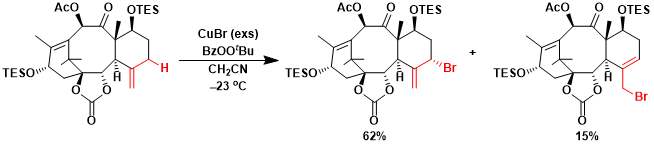
The application to the synthesis of Taxol.[1]
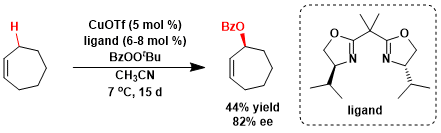
An asymmetric version using a chiral ligand.[2]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Mukaiyama, T. et al. Chem. Eur. J. 1999, 5, 121. [abstract]
[2] Gokhale, A. S.; Minidis, A. B. E.; Pfaltz, A. Tetrahedron Lett. 1995, 36, 1831. doi:10.1016/0040-4039(95)00140-8
-
Related Reactions
- Fenton Reaction
- Catalytic C-H Oxidation
- Selenium Dioxide
-
Related Books
[amazonjs asin=”0198556640″ locale=”US” title=”Oxidation and Reduction in Organic Synthesis (Oxford Chemistry Primers)”]
[amazonjs asin=”3527323201″ locale=”US” title=”Modern Oxidation Methods”]
[amazonjs asin=”1119953278″ locale=”US” title=”Handbook of Reagents for Organic Synthesis: Catalytic Oxidation Reagents”]
[amazonjs asin=”1466506016″ locale=”US” title=”Oxidation in Organic Synthesis”]
-
External Links
- Kharasch reaction and related transformation (Baran’s group, PDF)
- Kharasch-Sosnovsky Reaction – Wikipedia