- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
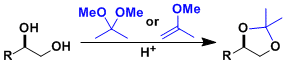
1,2- and 1,3-diols are generally protected as 5- or 6-membered cyclic acetal compounds.
-
General References
・Ley, S. V.; Baeschlin, D. K.; Dixon, D. J.; Foster, A. C.; Ince, S. J.; Priepke, H. W. M.; Reynolds, D. J. Chem. Rev. 2001, 101, 53. DOI: 10.1021/cr990101j
- Reaction Mechanism
- Examples
Regioelective protection of D-mannitol.[1]
Regioselective protection of methyl glucose.[2]
-
Experimental Procedure
-
Experimental Tips
Common protective groups are shown below. Benzylidene acetals and dimethyl acetals have thermodynamic tendency to form six- and five-membered rings, respectively. This can be utilized in the regioselective protection of polyol compounds.
Acetals are stable under basic and reductive conditions and unstable toward acids. Carbonates are deprotected under basic conditions. Silyl acetals are usually deprotected with fluoride sources.
- References
[1] Schmid, C. R.; Bryant, J. D. Org. Synth. 1995, 72, 6.
[2] Ferro, V.; Mocerino, M.; Stick, R. V.; Tilbrook, D. M. G. Aust. J. Chem. 1988, 41, 813.
- Related Books
[amazonjs asin=”0471697540″ locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”]
[amazonjs asin=”1588903761″ locale=”US” title=”Protecting Groups (softcover)”]

![PG_diol_4[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_diol_41.gif)
![PG_diol_5[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_diol_51.gif)
![PG_diol_2[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_diol_21.gif)