- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
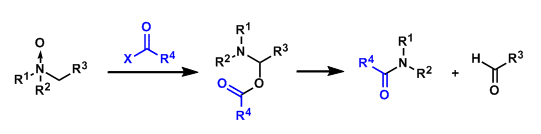
The acylation of amine N-oxides with acid chlorides or acid anhydrides leads to bond fragmentation at the α-position, yielding the corresponding amides and aldehydes as products.
In particular, treatment with trifluoroacetic anhydride (TFAA) is known to promote the reaction under mild conditions (the Potier modification).
This reaction is often used for demethylation of amines and oxidation of the α-position of pyridines.
-
General References
Polonovski, M.; Polonovski, M. Bull. Soc. Chim. France 1927, 41, 1190.
Polonovski, M. Bull. Soc. Chim. Belg. 1930, 39, 1.
Lounasmaa, M.; Koskinen, A. Heterocycles 1984, 11, 1591.
Grierson, D. Org. React. 1990, 39, 85.
< Potier Modification >
Cave, A.; Kan-Fan, C.; Potier, P.; Le Men, J. Tetrahedron 1967, 23, 4681. doi:10.1016/S0040-4020(01)92566-9
Tamminen, T.; Jokela, R.; Tirkkonen, B.; Lounasmaa, M. Tetrahedron 1989, 45, 2683. doi:10.1016/S0040-4020(01)80098-3
Sundberg, R. J.; Gadamasetti, K. G.; Hunt, P. J. Tetrahedron 1992, 48, 277. doi:10.1016/S0040-4020(01)88140-0
-
Reaction Mechanism
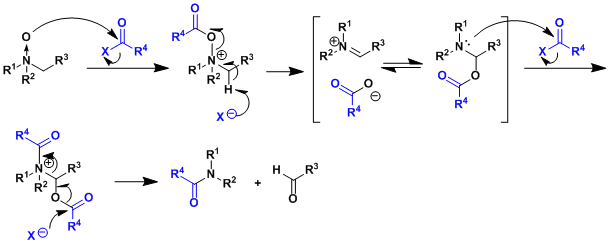
The reaction mechanism resembles that of the Pummerer rearrangement.
-
Examples
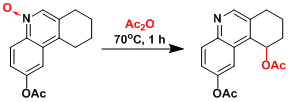
An example in the total synthesis of dynemicin A.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Shair, M. D.; Yoon, T. Y.; Mosny, K. K.; Chou, T. C.; Danishefsky, S. J. J. Am. Chem. Soc. 1996, 118, 9509. DOI: 10.1021/ja960040w
-
Related Reactions
-
Related Books
-
External Links