- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
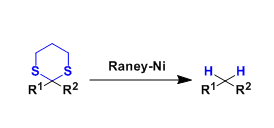
Thioethers and thioacetals can be reductively desulfurized by treating them with Raney nickel. The reduction of the latter is a common way to deoxygenate carbonyl groups.
Similar desulfurization can be done under the Barton-McCombie free-radical conditions (AIBN, Bu3SnH).
-
General References
- Pettit, G. R.; van Tamelen, E. E. Org. React.1962, 12, 356.
- Free radical desulfurization: Gutierrez, C. G.; Stingham, R. A.; Nitasaka, T.; Glasscock, K. G. J. Org. Chem. 1980, 45, 3393. DOI: 10.1021/jo01305a004
-
Reaction Mechanism
The reductant is the hydrogen adsorbed on the metal surface during the preparation of Raney nickel.
-
Examples
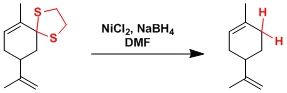
A typical example of the convertion of ketone to methylene group by desulfurization of thioacetal.
-
Experimental Procedure
-
Experimental Tips
One should be careful that the reactivity of Raney nickel often differs depending on how it is prepared, and that Raney nickel is pyrophoric when it is dried.
The use of nickel boride Ni2B (= NiCl2 + NaBH4) is a way to effect the same transformation avoiding these problems. Olefins are not reduced either under these conditions.[1]
-
References
[1] (a) Brown, C. A. J. Org. Chem. 1970, 35, 1900. DOI: 10.1021/jo00831a039 (b) Brown, C. A.; Ahuja, V. K. J. Org. Chem. 1973, 38, 2226. DOI: 10.1021/jo00952a024
[2] (a) Boar, R. B.; Hawkins, D. W.; McGhie, J. F.; Barton, D. H. R. J. Chem. Soc. Perkin Trans. 1973, 654. (b) Zaman, S. S.; Sarmah, P.; Barua, N. C. Chem. Ind. 1989, 806 .
-
Related Reactions
- Marko-Lam Deoxygenation
- Barton Decarboxylation
- Tsuji-Wilkinson Decarbonylation
- Ether Synthesis by Reduction of Acetal
- 1,3-Dithiane
- Lawesson’s Reagent
- Deoxofluorination
- Barton-McCombie Deoxygenation
- Clemmensen Reduction
- Conversion from Alcohol to Alkane
- Fukuyama Reduction
- Wolff-Kishner Reduction
-
Related Books
-
External Links