Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
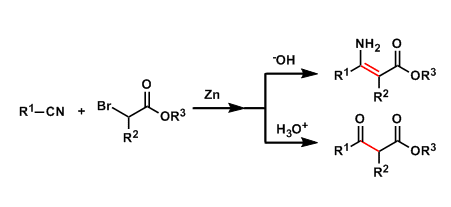
In the Blaise reaction, nitriles and zinc-enolates (prepared from zinc and α-haloesters) react to give β-enaminoesters or β-ketoesters.
-
General References
- Blaise, E. E. Compt. Rend. 1901, 132, 478.
- Cason, J.; Rinehart, K. L.; Thornton, S. D. J. Org. Chem. 1953, 18, 1594. doi:10.1021/jo50017a022
- Rinehart, K. L., Jr. Org. Synth. 1955, 35, 15. DOI: 10.15227/orgsyn.035.0015
- Hannick, S. M.; Kishi, Y. J. Org. Chem. 1983, 48, 3833. doi:10.1021/jo00169a053
- Prakash, H. S.; Rafia, S.; Padmavathy, K. Tetrahedron, 2008, 65, 8037. doi:10.1016/j.tet.2008.05.109
-
History
This reaction was reported by the French chemist Edmond Blaise in 1901. In 1983, Kishi et al. reported modifications of this reaction for their total synthesis of saxitoxin (1. The use of THF, 2. Preactivation of zinc, 3. Slow addition of α-bromoester).
-
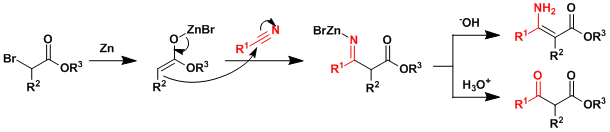
Reaction Mechanism
The reaction is nucleophilic addition of zinc enolate to nitrile. Note the similarity to the Reformatsky reaction.
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
- Claisen Condensation
- Reformatsky Reaction
-
Related Books
[amazonjs asin=”3527332057″ locale=”US” title=”Modern Methods in Stereoselective Aldol Reactions”]
-
External Links
- BlaiseReaction (organic-chemistry.org)
- Blaise Reaction– Wikipedia