- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
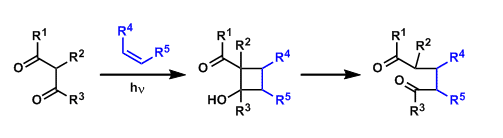
In the de Mayo reaction, 1,3-dicarboyl compounds and alkenes undergo photo-activated [2+2] cycloaddition. The subsequent ring opening gives 1,5-dicarbonyl products.
-
General References
- de Mayo, P. et al. Proc. Chem. Soc. London 1962, 119.
- de Mayo, P.; Takeshita, H. Can. J. Chem. 1963, 41, 440.
- Challand, B. D.; Hikino, H.; Kornis, G.; Lange, G.; de Mayo, P. J. Org. Chem. 1969, 34 , 794. DOI: 10.1021/jo01256a006
- de Mayo, P. Acc. Chem. Res. 1971, 4, 41. DOI: 10.1021/ar50038a001
<Photochemical reactions in total synthesis>
- Hoffmann, N. Chem. Rev. 2008, 108, 1052. DOI: 10.1021/cr0680336
- Bach, T.; Hehn, J. P. Angew. Chem. Int. Ed. 2011, 50, 1000. DOI: 10.1002/anie.201002845
-
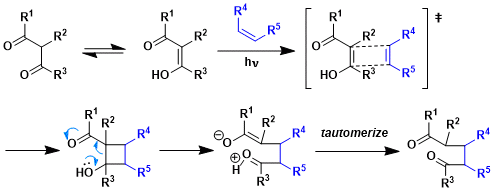
Reaction Mechanism
-
Examples
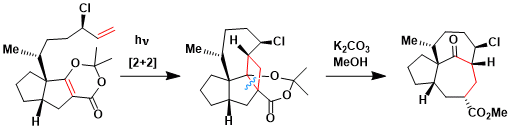
An application to the total synthesis of ingenol, where the fused 7-7-5 ring system was constructed by the cycloaddition-fragmentation sequence.[1]
-
Experimental Procedure
An example in which benzyne is used for the thermally activated cycloaddition.[2]
An oven-dried 500-mL, three-necked, round-bottomed flask is equipped with a magnetic stir bar, and cesium fluoride (19.7 g, 130 mmol, 2.5 equiv) is added. The flask is fitted with a reflux condenser in the middle neck, an adaptor equipped with a thermometer in one of the side necks, and a septum in the other side neck. The condenser is equipped with a vacuum adaptor connected to a Schlenk line (nitrogen/vacuum manifold). The connected glassware is evacuated under high vacuum (0.025 mmHg) and carefully back-filled with nitrogen. This procedure is repeated twice to secure an oxygen-free atmosphere in the flask. Dry MeCN (260 mL) is added to the flask via a syringe through the septum. While stirring the reaction mixture, methyl acetoacetate (5.60 mL, 6.01 g, 51.8 mmol, 1.00 equiv) and 2-(trimethylsilyl)phenyl trifluoromethanesulfonate (15.7 mL, 19.3 g, 64.7 mmol, 1.25 equiv) are added through the septum via syringes. The flask is submerged in an oil bath (100 °C) and the reaction mixture is heated to reflux (internal temperature: 78-81 °C, reached after 15 min). The mixture is stirred at reflux temperature for 40 min. Initially, the reaction mixture is a white opaque suspension, but during heating it changes color to yellow and upon reflux the color changes to orange. As the reaction progresses the opaque solution becomes transparent and the color changes back to yellow. Throughout the entire time a white precipitate is present in the flask. The reaction flask is removed from the oil bath and allowed to cool to ambient temperature (23 °C) over the course of 1 h. The flask is disconnected from the condenser and the nitrogen inlet, and the reaction mixture is diluted with saturated aqueous NaCl solution (200 mL). This mixture is carefully poured into a 1-L separatory funnel. After the layers are separated, the aqueous layer is extracted with Et2O (3 × 200 mL). The combined organic layers are dried over anhydrous Na2SO4 and filtered. Concentration of the dried organic layers is effected by rotary evaporation (35 °C, 45 mmHg) which affords an orange, viscous oil. Partial purification is achieved by flash chromatography (4.5 × 24 cm, 170 g silica gel) using a gradient of hexanes to 40% Et2O in hexanes. The crude product is loaded on the column with benzene (20 mL). Fraction collection (50 mL fractions) is begun as the crude product is eluted first with 100 mL of hexanes, followed by 1500 mL of 9:1 hexanes:Et2O, 2000 mL of 4:1 hexanes:Et2O, 1000 mL of 7:3 hexanes:Et2O and finally 750 mL of 3:2 hexanes:Et2O. Fractions 38-85 are concentrated by rotary evaporation (30-35 °C, 45 mmHg) to afford 7.96 g (80%) of a slightly yellow solid. The partially purified product is further purified by bulb-to-bulb distillation at 124-130 °C (0.75 mmHg) which affords 6.63 g (67%) of the title compound as an off-white, crystalline solid.
-
Experimental Tips
-
References
[1] Winkler, J. D.; Rouse, M. B.; Greaney, M. F.; Harrison, S. J.; Jeon, Y. T. J. Am. Chem. Soc. 2002, 124, 9726. doi:10.1021/ja026600a
[2] Ebner, D. C.; Tambar, U. K.; Stoltz. B. M. Org. Synth. 2009, 86, 161. [website]
-
Related Reactions
- Di-π-methane Rearrangement
- Benzyne
- [2+2] Cycloaddition of Ketene
- [6π]Photocyclization
- Paterno-Büchi Reaction
- [2+2] Photocyclization
-
Related Books
[amazonjs asin=”1891389572″ locale=”US” title=”Principles of Molecular Photochemistry: An Introduction”]
-
External Links