Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
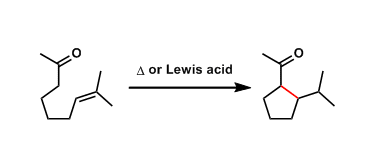
In the Conia-ene reaction, the enol and the alkene or alkyne parts of an unsaturated carbonyl compound react and undergo intramolecular cyclization.
-
General References
- Rouessac, F. Tetrahedron Lett. 1965, 6, 3319. doi:10.1016/S0040-4039(01)89202-9
- Conia, J. M., Le Perchec, P. Synthesis 1975, 1. doi:10.1055/s-1975-23652
- Kende, A. S.; Newbold, R. C. Tetrahedron Lett. 1989, 30, 4329. doi:10.1016/S0040-4039(00)99352-3
-
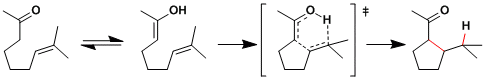
Reaction Mechanism
-
Examples
In 2004, Toste reported the gold-catalyzed Conia-ene type reaction of β-ketoesters containing unactivated alkyne.[1] The enantioselective version of this reaction using Pd(II)/Yb(III) dual catalyst system was reported a year later.[2]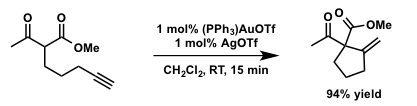
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Kennedy-Smith, J. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 4526. DOI: 10.1021/ja049487s[2] Corkey, B. K.; Toste, F. D. J. Am. Chem. Soc., 2005, 127, 17168. DOI: 10.1021/ja055059q
- Gao, Q.; Zheng, B. -F.; Li, J.-H.; Yang, D. Org. Lett. 2005, 7, 2185. DOI: 10.1021/ol050532q
- Corkey, B. K.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 17168. DOI: 10.1021/ja055059q
- Urabe, F.; Nagashima, S.; Takahashi, K.; Ishihara, J.; Hatakeyama, S. J Org Chem 2013, 78, 3847. DOI: 10.1021/jo400263w
-
Related Reactions
-
Related Books
[amazonjs asin=”3642692354″ locale=”US” title=”Intramolecular Diels-Alder and Alder Ene Reactions (Reactivity and Structure: Concepts in Organic Chemistry)”]
-
External Links
- Conia-Ene Reaction (organic-chemistry.org)
- Ene Reaction- Wikipedia
- Catalytic Enantioselective Conia-Ene Reaction (organic-chemistry.org)