Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
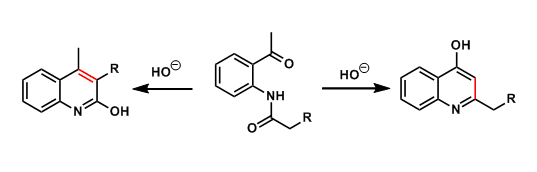
The basic treatment of ortho-acylaminoacetophenones leads to cyclocondensation, producing hydroxyquinolines. The experimenter needs to be aware of possible isomer formation.
-
General References
- Camps, R. Ber. 1899, 22, 3228.
- Camps, R. Arch. Pharm. 1899, 237, 659.
- Camps, R. Arch. Pharm. 1901, 239, 591.
- Manske, R. H. F. Chem. Rev. 1942, 30, 127.
-
Reaction Mechanism
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
- Povarov Reaction
- Pomeranz-Fritsch Isoquinoline Synthesis
- Conrad-Limpach Quinoline Synthesis
- Combes Quinoline Synthesis
- Niementowski Quinoline/Quinazoline Synthesis
- Bischler-Napieralski Isoquinoline Synthesis
- Pfitzinger Quinoline Synthesis
- Skraup Quinoline Synthesis
- Knorr Quinoline Synthesis
- Friedländer Quinoline Synthesis
- Pictet-Gams Isoquinoline Synthesis
- Doebner-von Miller quinoline synthesis
- Pictet-Spengler Reaction
-
Related Books
[amazonjs asin=”0470085088″ locale=”US” title=”Name Reactions in Heterocyclic Chemistry II”]
-
External Links