Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
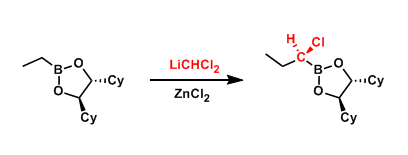
The one-carbon homologation of boronate compounds using α-halocarbanions is called the Matteson reaction. The rearrangement of the intermediate can be rendered stereoselective if the substrate has a chiral auxiliary.
-
General References
- Matteson, D. S.; Mah, R. W. H. J. Am. Chem. Soc. 1963, 85, 2599. DOI: 10.1021/ja00900a017
- Rathke, M. W.; Chao, E.; Wu, G. J. Organomet. Chem. 1976, 122, 145. doi:10.1016/S0022-328X(00)80606-3
- Matteson, D. S.; Majumdar, D. J. Am. Chem. Soc. 1980, 102, 7588. DOI: 10.1021/ja00545a045
- Matteson, D. S.; Ray, R. J. Am. Chem. Soc. 1980, 102, 7590. DOI: 10.1021/ja00545a046
- Matteson, D. S.; Man, H.-W.; Ho, O. C. J. Am. Chem. Soc. 1996, 118, 4560. DOI: 10.1021/ja960345a
- Review: Matterson, D. S. Tetrahedron 1989, 45, 1859. doi:10.1016/S0040-4020(01)80052-1
- Review: Matterson, D. S. Tetrahedron 1998, 54, 10555. doi:10.1016/S0040-4020(98)00321-4
-
Reaction Mechanism
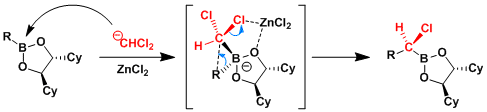
Ref: Tetrahedron: Asymmetry 1997, 8, 3711.
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
-
Related Books
-
External Links