Overall Score5
- Generality
- Importance as Chemical Principle
- Criteria #3
- Criteria #4
- Criteria #5
-
General Characteristics
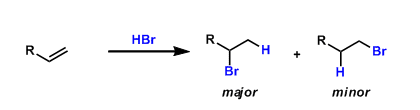
In electrophilic addition reactions of alkenes, the electrophiles (electronically positive atoms or groups) generally bond with the carbon that has more hydrogen atoms. This empirical rule is known as the Markovnikov’s rule.
A useful way to remember it is “the rich get richer,” as the alkenyl carbon having more hydrogen atoms gets more (proton or other electrophiles).
-
General References
- Markovnikov, W. Ann. Pharm. 1870, 153, 228. DOI:10.1002/jlac.18701530204
- Peter, H. J. Chem. Edu. 2006, 83, 1152. DOI:10.1021/ed083p1152
-
Reaction Mechanism
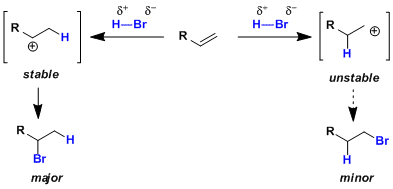
The Markovnikov’s rule is explained based on the relative stability of carbocationic intermediates. When proton or other electrophile adds to the less substituted side of the two carbons, the resulting carbocation is more stabilized by hyperconjugation, which is then captured by the nucleophile.
-
Related Reactions
-
External Links
- Markovnikov’s Rule – Wikipedia
- Markovnikov’s rule (organic-chemisry.org)
- Addition to Alkene: regiochemistry